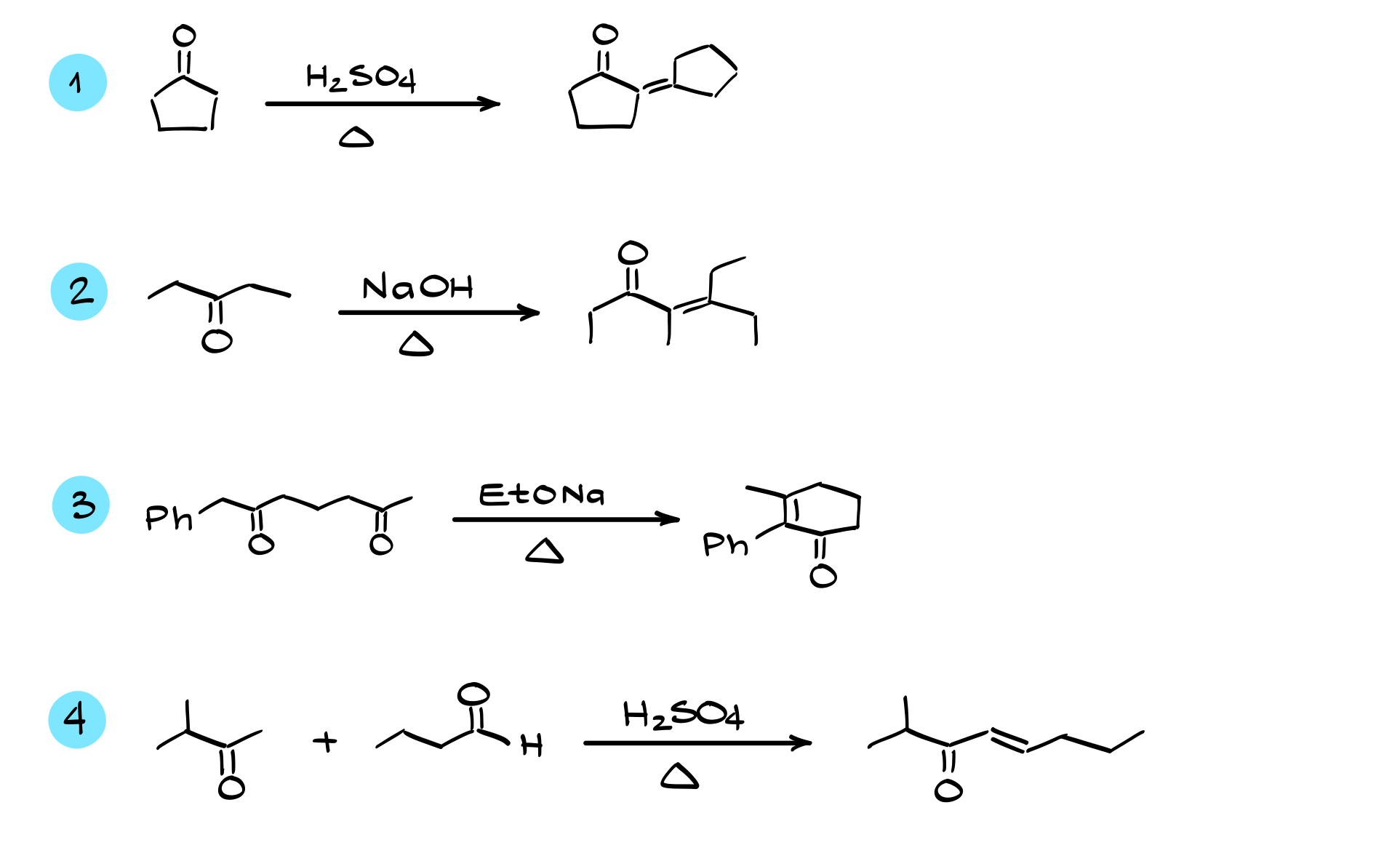
Aldol Condensation Practice Questions
In this tutorial, I’m going to work through a few aldol reaction practice questions and then show you a trick that will allow you to quickly predict the products of any aldol condensation reaction. So make sure you stick around until the very end. Without further ado, grab your notebook, and let’s get started.
Example 1
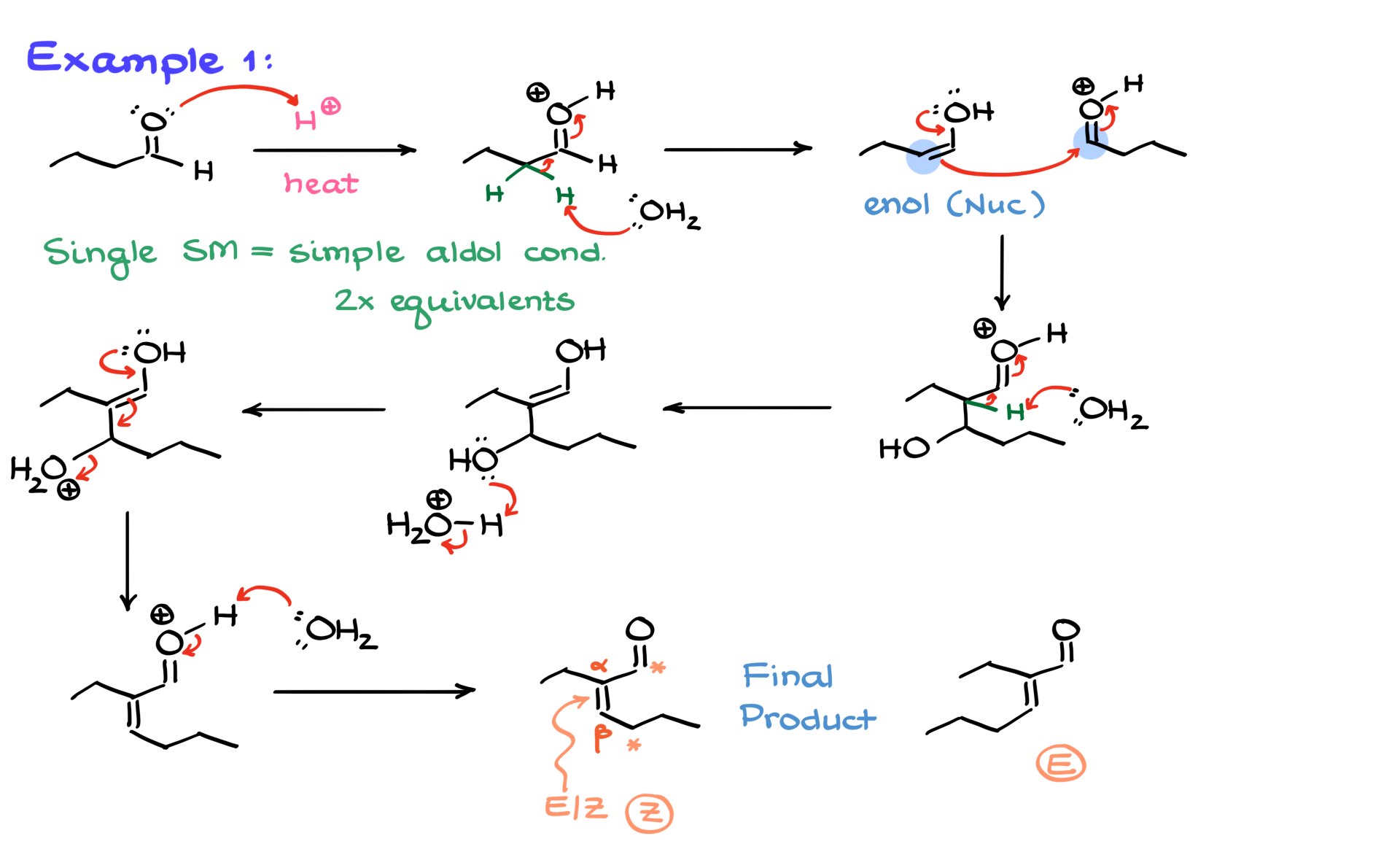
In the first example, we have an aldehyde as our starting material, and we’re running the reaction under acidic conditions at an elevated temperature. Since we only have one starting material, we’re looking at a simple aldol condensation reaction. We know it’s a condensation reaction because of the elevated temperature, meaning we’re going all the way to form the α,β-unsaturated compound. Also, since there’s only one starting material, it means the reaction is happening between two equivalents (or two molecules) of that same compound. While some instructors may explicitly write “2 equivalents” in the problem, it’s typically assumed.
The first step in this reaction is the protonation of the carbonyl, giving us an intermediate that we can deprotonate at the α-position. Typically, water will pull off that proton, leading to the formation of the corresponding enol. To keep my diagram clear, I won’t show the implicit hydrogen here—it’ll be easier to follow the bonds we’re forming. Now that we have our enol, we recognize that it’s the nucleophile in this reaction. The next step involves bringing in our electrophile, which is the second equivalent of our aldehyde. To make the transition smoother, I’ll show the protonated version of my electrophile right away.
At this stage, our nucleophilic enol attacks the electrophilic carbonyl, forming a new carbon-carbon bond between the α-carbon and the carbonyl carbon. Since we’re going all the way to the α,β-unsaturated compound, we won’t isolate the aldol product but will proceed directly to enolization. This means we’ll deprotonate at the α-position again, forming another enol. Another equivalent of water then protonates the hydroxyl group, making it a better leaving group. Finally, the enol pushes off the leaving group, yielding the protonated version of our α,β-unsaturated product. This compound quickly loses a proton to the surroundings, giving us the final product.
Now, the double bond in the final product can be either E or Z, depending on the molecular structure. In this particular case, the way I drew it corresponds to the Z stereoisomer. If we wanted, we could also draw the E stereoisomer. While stereochemistry isn’t always the focus in aldol condensation reactions, some instructors might include a follow-up question asking for the specific stereochemistry or requiring you to assign the configuration. But other than that, this is our final product. Now, let’s move on to the next problem.
Example 2
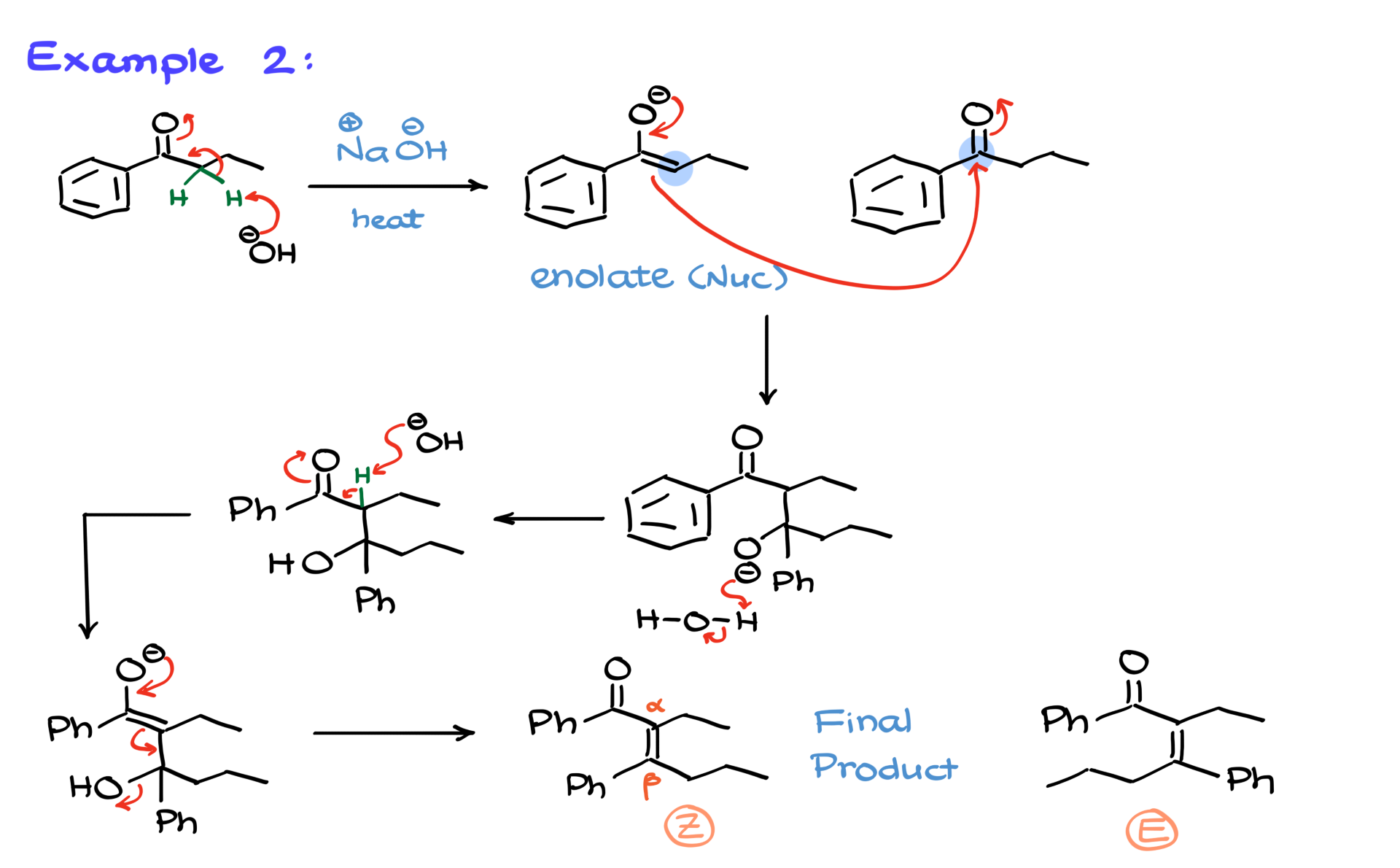
In the second example, we’re working with an aromatic ketone under basic conditions at an elevated temperature, so once again, we’re dealing with aldol condensation. However, this time the reaction is in basic media, meaning we start by deprotonating the α-position of the ketone to form the enolate. Once we have our enolate, it reacts with a second equivalent of the ketone, forming a new carbon-carbon bond between the α-carbon and the carbonyl carbon.
To make things clearer, I abbreviated one of my aromatic rings as “Ph” (for phenyl) to avoid overcrowding the diagram. If you wanted, you could draw the full structure, but I’ll stick with the abbreviation for simplicity. After forming the aldol intermediate, we protonate the negatively charged oxygen using water, giving us the β-hydroxy ketone. From here, we bring back our base to deprotonate at the α-position, forming another enolate. This enolate then eliminates the hydroxyl group as water, yielding the α,β-unsaturated carbonyl product.
Again, I’ve drawn the Z stereoisomer, but if you drew the E stereoisomer, that would be equally correct. The exact stereochemistry isn’t the focus here, but some instructors might ask for it.
Example 3
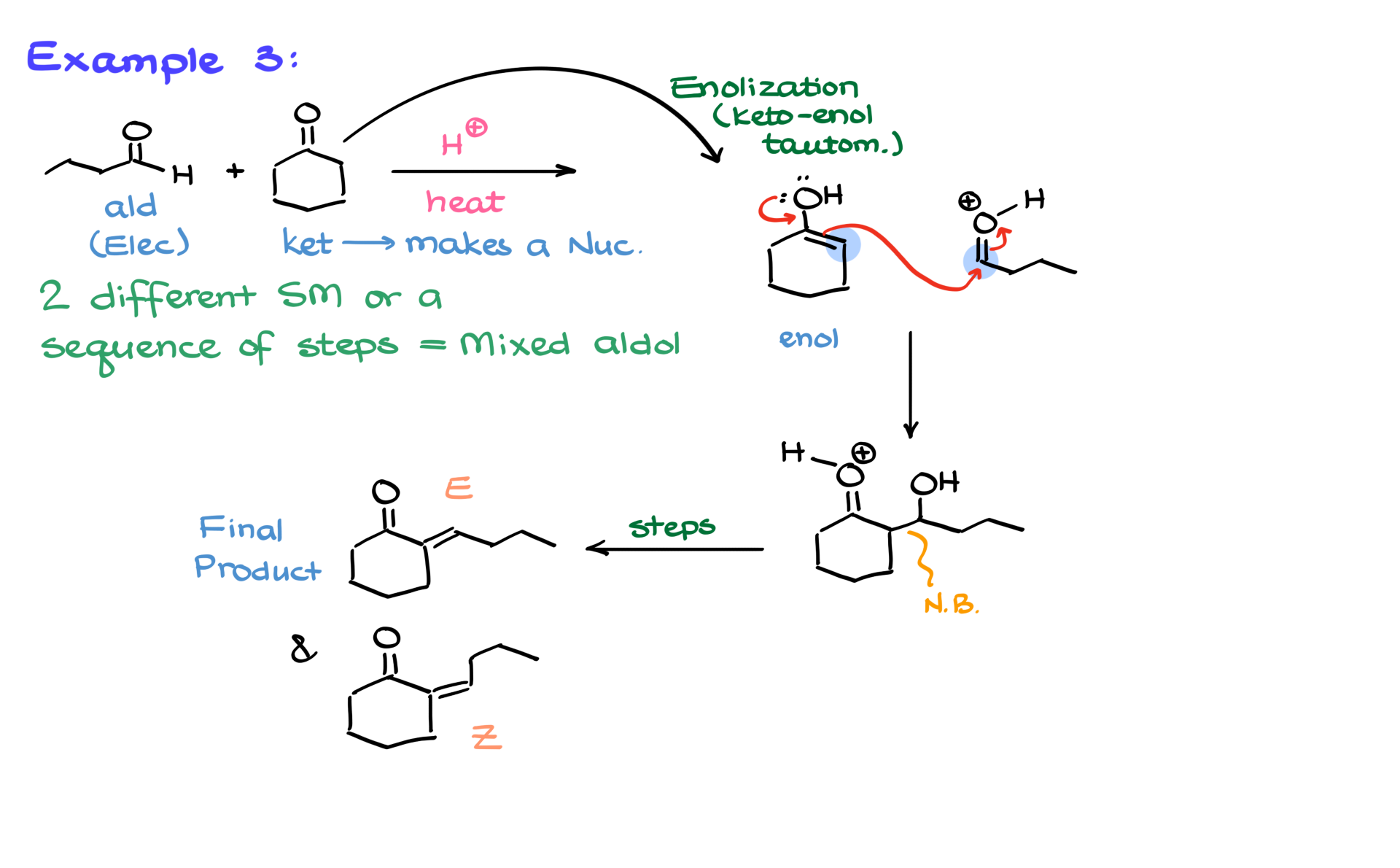
Moving on to the next example, we now have a mixed aldol condensation, meaning we’re reacting two different carbonyl compounds—an aldehyde and a ketone—under acidic conditions. Since aldehydes are more electrophilic than ketones, the aldehyde will act as the electrophile while the ketone forms the nucleophilic enol.
First, we form the enol from the ketone via keto-enol tautomerism. Then, this enol reacts with the protonated aldehyde, forming a new carbon-carbon bond between the α-carbon of the enol and the aldehyde’s carbonyl carbon. The rest of the mechanism follows the same steps as before: enolization, protonation of the hydroxyl group, and elimination of water to form the final α,β-unsaturated product. In this case, the E stereoisomer is the major product, but the Z isomer could also form.
Now, since this is a mixed aldol condensation, multiple side products are possible, but for exam purposes, you only need to draw the major product—the E or Z isomer—and ignore the minor ones.
Example 4
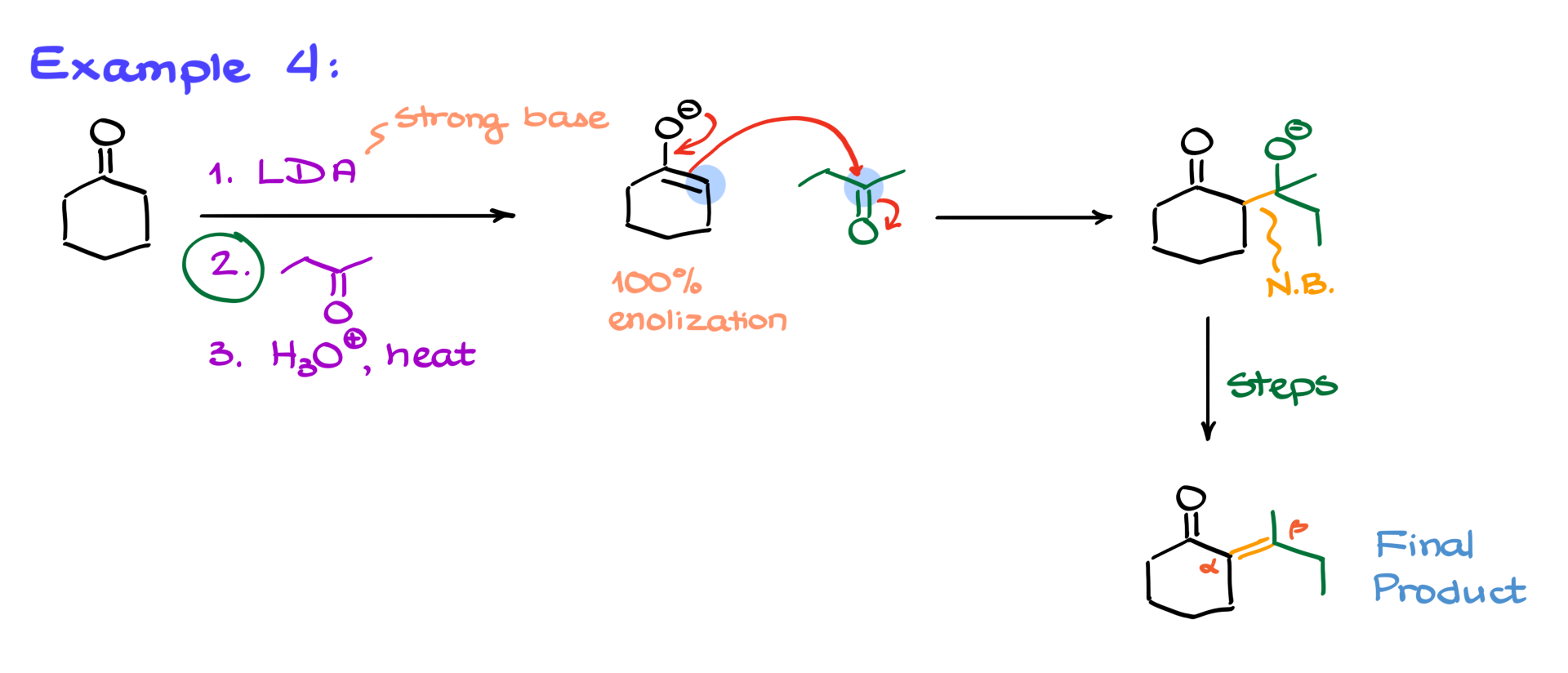
For our next reaction, we have a stepwise process, meaning we can control which enolate forms and avoid unwanted side products. Our first reagent is LDA (lithium diisopropylamide), a strong base that ensures complete enolization of the ketone. This gives us an enolate that reacts with the second carbonyl compound in step two. Following the same pattern, the enolate attacks the carbonyl, forming a new C–C bond. After protonation, elimination leads to the α,β-unsaturated product.
Once you get comfortable with these mechanisms, you’ll find that you don’t need to write out every single step. Instead, you can visualize the major intermediates and mentally work through the rest. This approach speeds things up significantly, especially on exams.
Example 5
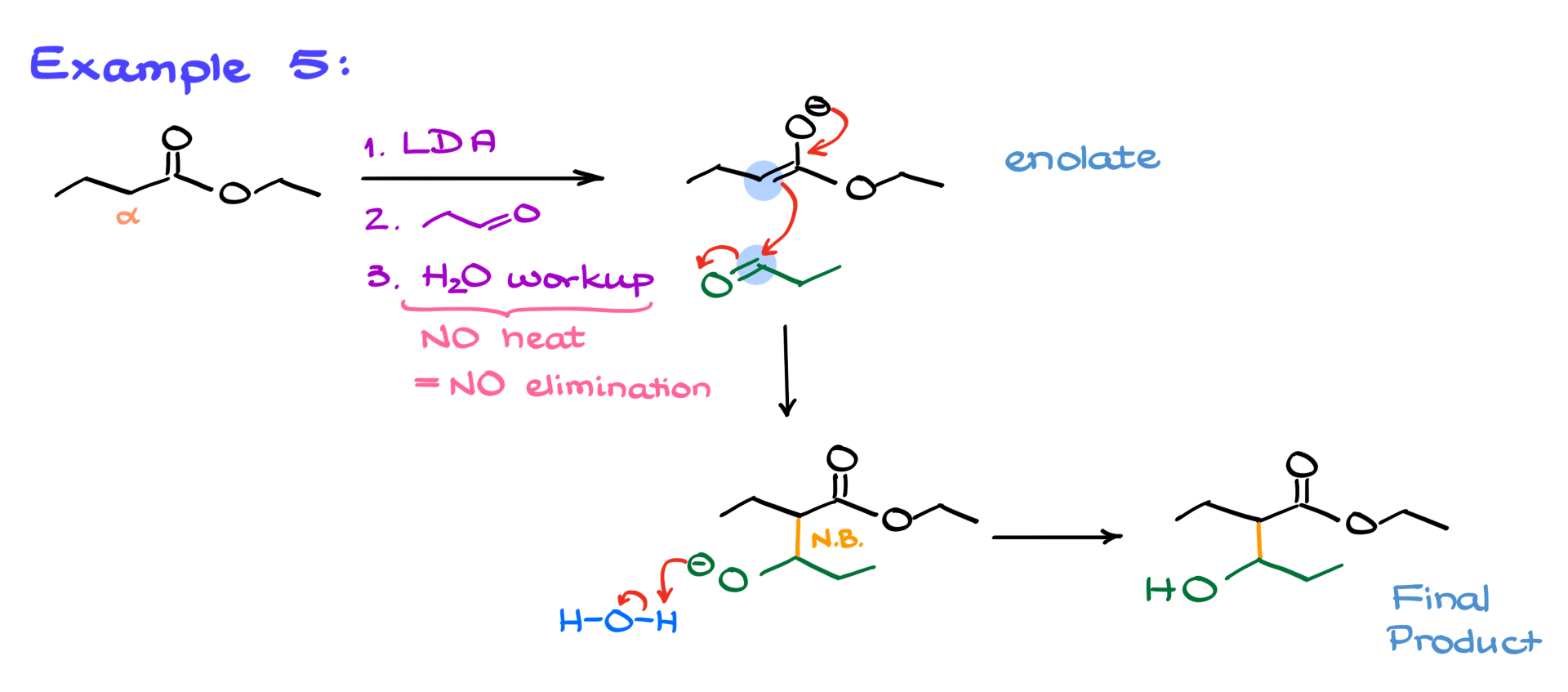
The same principle applies when working with esters. Although esters have slightly less acidic α-hydrogens than ketones (pKa ~22-24 vs. ~19), using a strong base like LDA ensures enolate formation. The enolate then reacts with an aldehyde to form the aldol product. Since the problem states “no heat,” there’s no elimination—meaning we stop at the β-hydroxy carbonyl stage instead of forming the α,β-unsaturated compound.
One trick that makes predicting aldol condensation products much easier is color coding. By keeping track of different parts of the molecule in distinct colors, you can clearly see how the starting materials contribute to the final product.
Example 6
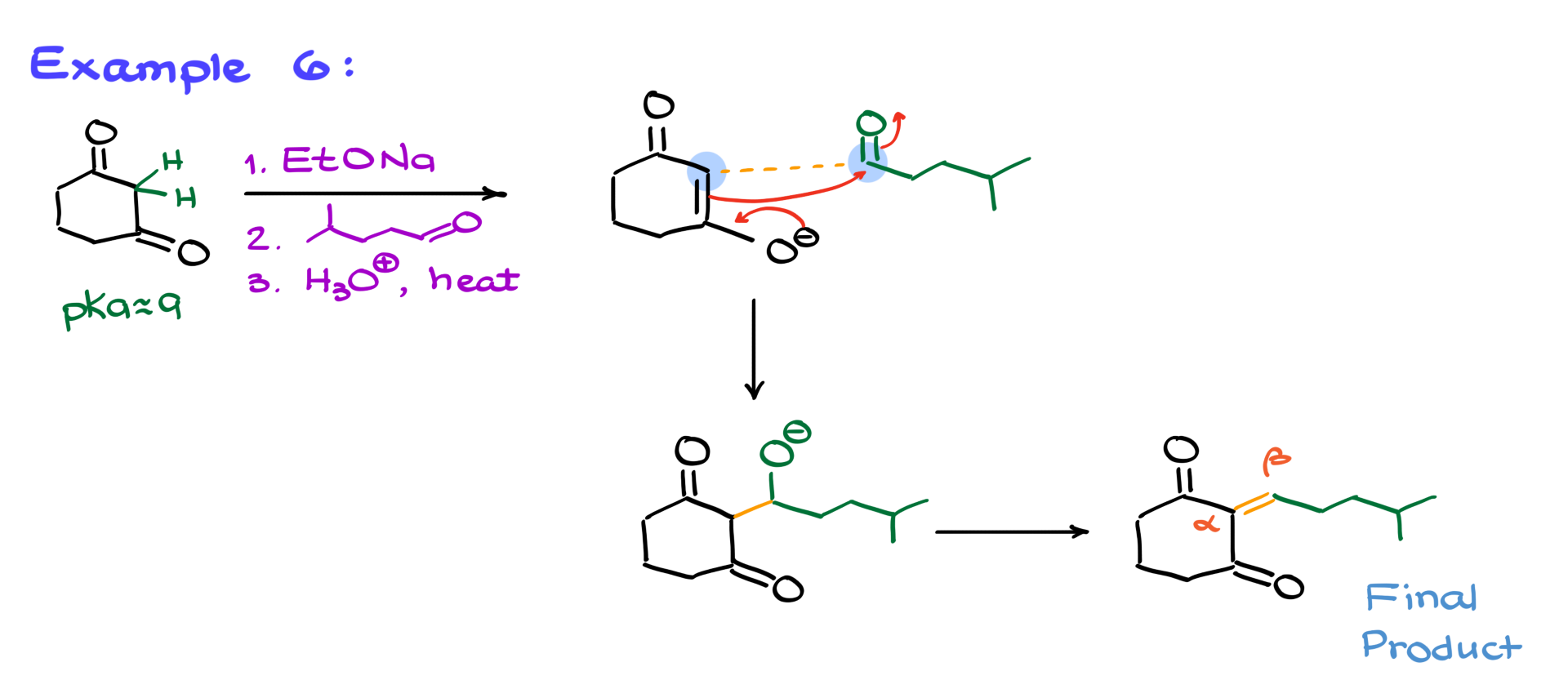
Let’s apply this technique to a diketone example. Since 1,3-diketones are significantly more acidic (pKa ~9), even a mild base like sodium ethoxide is enough to fully deprotonate them, forming an enolate. When this enolate reacts with an aldehyde, the reaction follows the same steps: nucleophilic attack, protonation, enolate formation, and elimination. The final product is the expected α,β-unsaturated compound.
Trick!
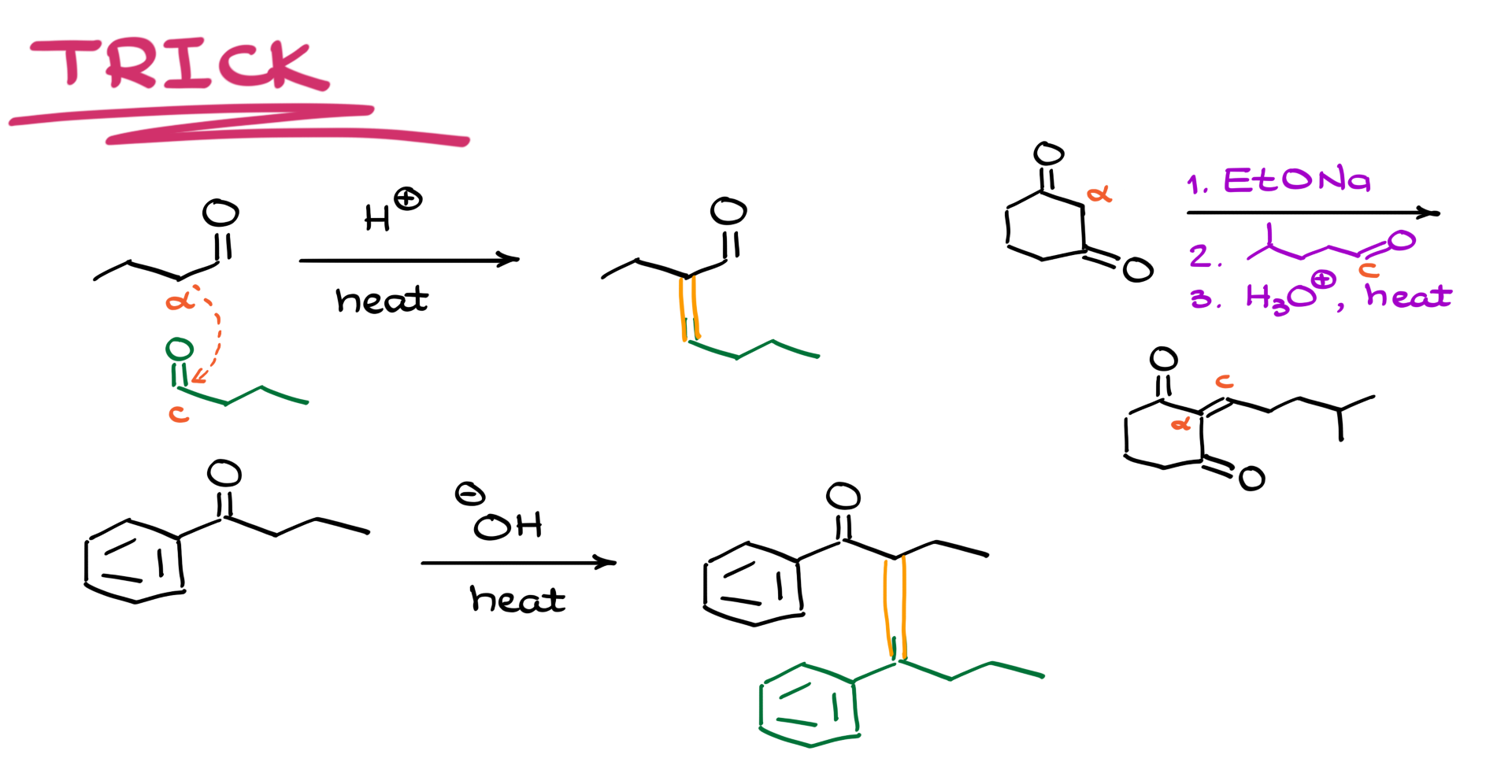
Now, let me show you how you can quickly predict the product of any aldol condensation without drawing out every single step.
1. Identify the α-position on one molecule and the carbonyl carbon on the other.
2. Draw them close together so the α-carbon aligns with the carbonyl carbon.
3. Erase the carbonyl oxygen and replace it with a double bond.
Boom! You’ve got your final product.
Let’s try this with an example:
• Take butanal, an aldehyde.
• Recognize that one molecule’s α-position reacts with another’s carbonyl.
• Draw them together, erase the oxygen, and replace it with a double bond.
That’s it! This trick works for any aldol condensation reaction, no matter how complex. It saves a ton of time on exams and homework while ensuring accuracy.
Of course, you still need to understand the full mechanism, but when you’re short on time, this method allows you to quickly and confidently predict aldol condensation products.
Practice Questions
Would you like to see the answers and check your work? Become a member today or login if you’re already a member and unlock all members-only content!