Aldol Condensation
In this tutorial, I want to start our exploration of the aldol condensation, which is an extremely important reaction of carbonyl compounds. Since aldol condensation is a fairly large and complex topic, I am going to break it into four parts. We will discuss aldol condensation under acidic conditions, explore it under basic conditions, examine the mixed aldol condensation separately, and finally, I will provide a dedicated tutorial with examples and a nifty trick that will allow you to predict the products of the aldol condensation quickly.
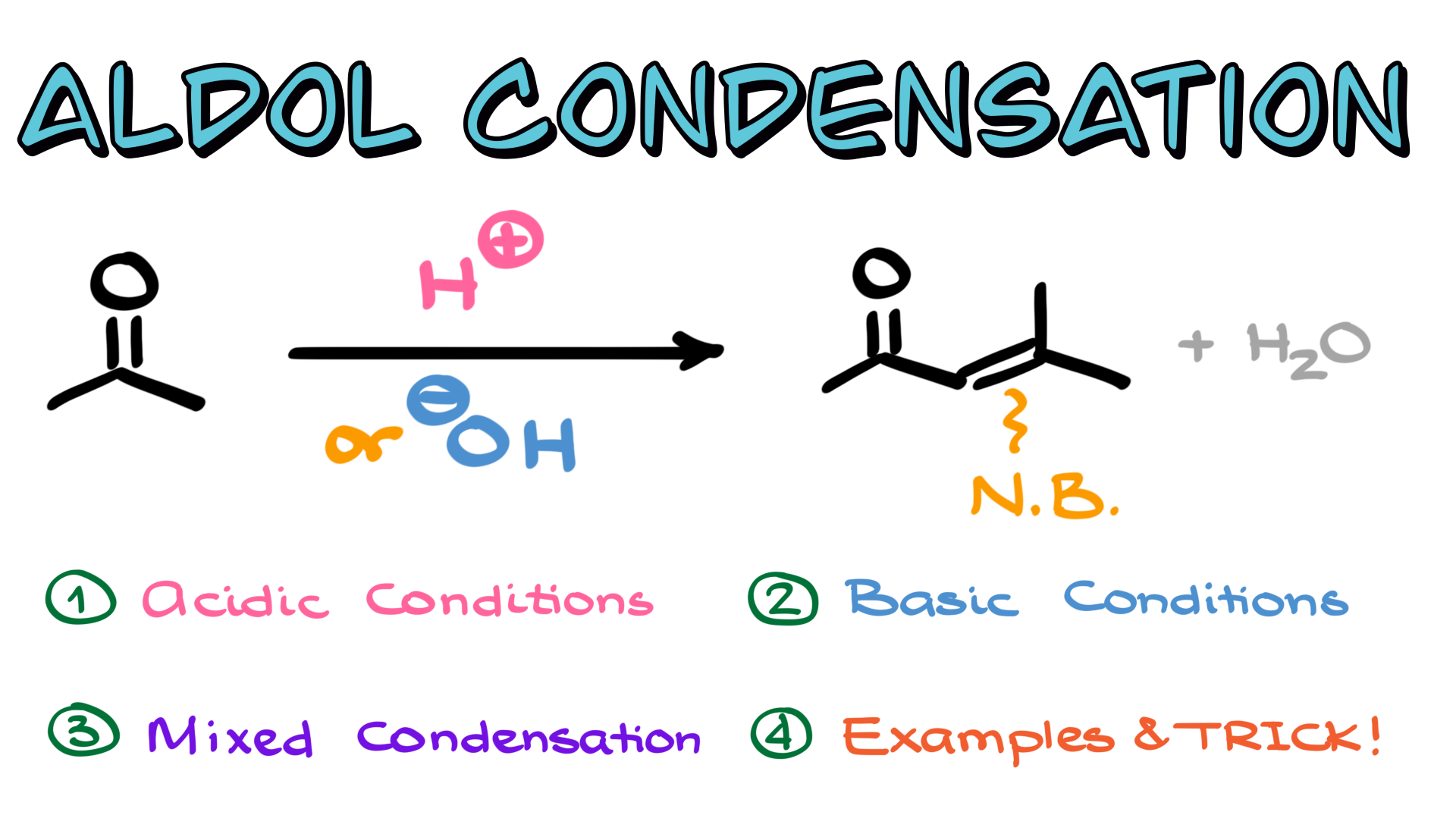
Aldol Condensation in Acidic Conditions
Generally speaking, aldol condensation is a reaction in which we take an aldehyde or ketone, treat it with acid, and initially form a β-hydroxy carbonyl compound. A new carbon-carbon bond is formed in this process, making it an essential reaction for carbon-carbon bond formation. The intermediate product, commonly referred to as an aldol, is where the reaction gets its name. However, this aldol may not always be the final product. If we supply heat, the reaction proceeds further through an elimination step, leading to the formation of a double bond. The resulting compound is referred to as an α,β-unsaturated carbonyl. You might also hear the term “ket-ene” (sometimes pronounced “ket-in”), where “ket” refers to the ketone portion and “en” signifies the double bond adjacent to it. Most commonly, the term ketene refers to compounds that look like R2C=C=O, so we typically use the more common term, α,β-unsaturated carbonyl, or simply α,β-unsaturated compound to avoid any confusion. Another common term you’re likely to hear associated with these compounds is the “enone.”

Mechanism of the Acidic Aldol Condensation
Now, let’s take a look at the mechanism of this reaction. Since we are working under acidic conditions, the very first step is the protonation of the carbonyl group, giving us an intermediate. This intermediate then loses a proton, usually to a conjugate base in the solution—for simplicity, I will just show water—resulting in the formation of an enol. At this point, we are simply observing keto-enol tautomerism in an acidic environment.
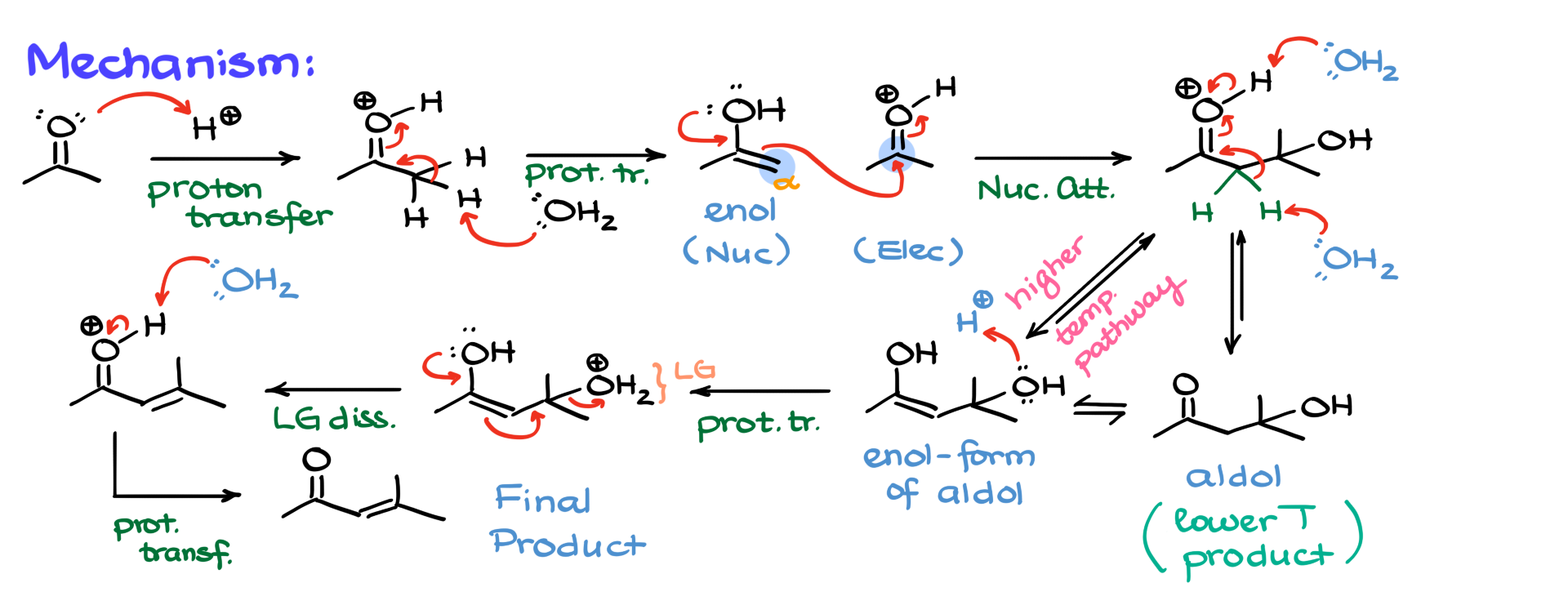
We know that enols are nucleophilic, and carbonyls are electrophilic. Since we are in acidic conditions, some of our carbonyl groups will be protonated, making them even stronger electrophiles. Since we have both a nucleophile and an electrophile in the same system, they will naturally react. The α-position of the enol attacks the carbonyl carbon of another molecule, forming a new carbon-carbon bond. This nucleophilic attack results in the formation of a protonated aldol intermediate.
At this stage, we have two possible pathways. The first option is to simply deprotonate the intermediate, forming the aldol or β-hydroxy carbonyl. The second option is to deprotonate at the α-position, leading to the enol form of the aldol. If the reaction is carried out at a lower temperature, it typically stops at aldol formation, and this product is referred to as the aldol addition product. However, at higher temperatures, the reaction continues. Since all of these steps are in equilibrium, drawing the aldol in different forms is not incorrect—it can always interconvert between these tautomers.
If the enol form of the aldol is present, one possible next step is the protonation of the hydroxyl group at the β-position, converting it into water, which is an excellent leaving group. At this point, the elimination step occurs, where the leaving group is expelled, forming a double bond between the α- and β-carbons. This final step yields our α,β-unsaturated carbonyl compound. The last step in the process is simply the deprotonation of the intermediate, resulting in the final α,β-unsaturated product.
Now, let’s quickly run through the mechanism one more time to reinforce the key steps.
- First, the carbonyl is protonated, forming a protonated carbonyl intermediate.
- This intermediate then deprotonates, leading to the formation of an enol, which acts as the nucleophile.
- The enol attacks the electrophilic carbonyl of another molecule, forming a protonated aldol.
- From here, either deprotonation at the oxygen produces the aldol product, or deprotonation at the α-position generates the enol form of the aldol.
- In acidic conditions, this enol can then be protonated at the hydroxyl group, creating a good leaving group, which is eliminated to form a double bond.
- The last step is the deprotonation of the intermediate, leading to the final α,β-unsaturated product.
Common Mistake
A very common mistake students make in this reaction appears at the elimination step, so I want to highlight it separately. When given an aldol product in acidic conditions with heat, it might be tempting to protonate the hydroxyl group, make it a leaving group, and proceed through carbocation formation before eliminating to form the double bond. However, this pathway is incorrect. Extensive experimental evidence shows that this reaction does not proceed through a carbocation intermediate. Instead, elimination occurs via enolization.

The correct pathway involves first protonating the carbonyl, converting it into its enol form. Only after enol formation does protonation of the hydroxyl group occur, leading to the creation of a leaving group. The enol then facilitates the elimination process through electron delocalization, ensuring that no carbocation is formed. This is a key distinction—this reaction does not follow an E1 elimination mechanism. Instead, the enol directly drives the elimination step.
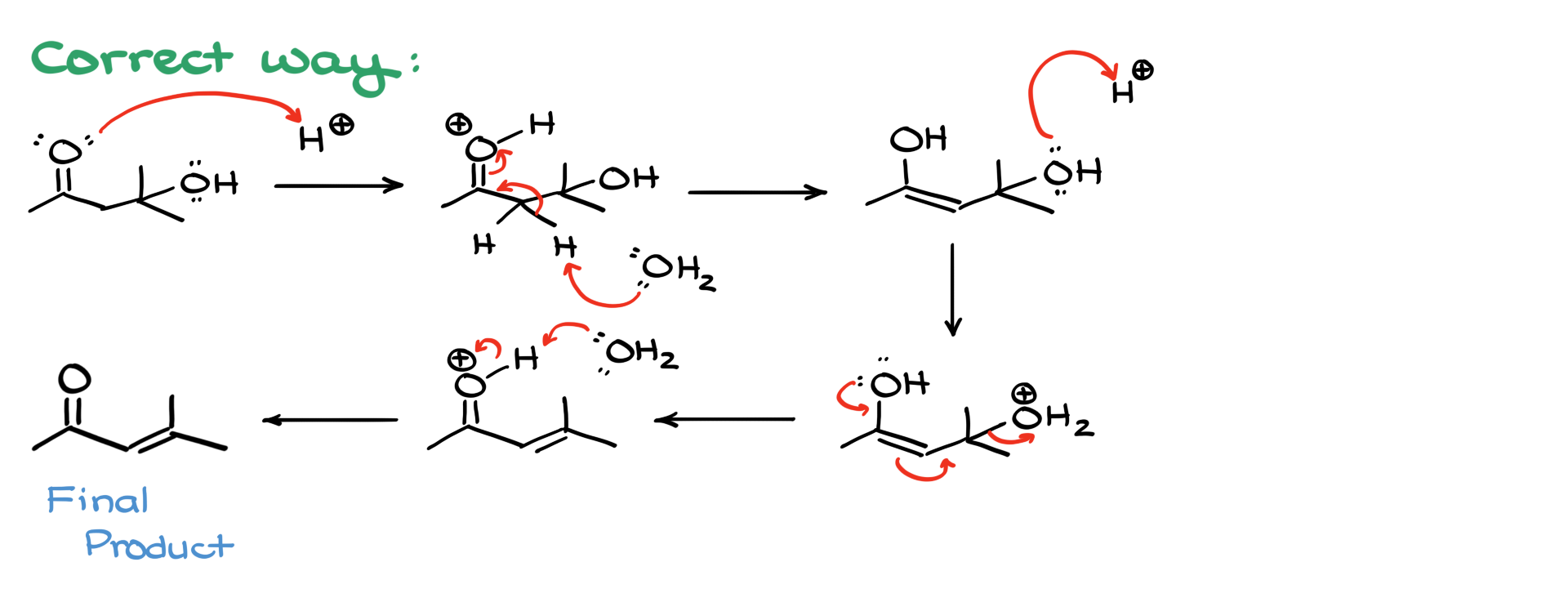
So, to summarize, remember that the aldol condensation in acidic media proceeds through enol formation and not through carbocation intermediates. This mechanism ensures the formation of a stable α,β-unsaturated product without the pitfalls of incorrect mechanistic assumptions. Now, let’s move on to the basic conditions!
Aldol Condensation in Basic Conditions
The general idea of aldol condensation in basic media is quite similar to what we saw in acidic conditions. We will still form an aldol intermediate, create a new carbon-carbon bond, and, if we run the reaction at an elevated temperature, we will end up with an α,β-unsaturated carbonyl as the final product. The key difference here is that instead of acidic conditions, we are working in basic conditions, which means our mechanism will be different as well.
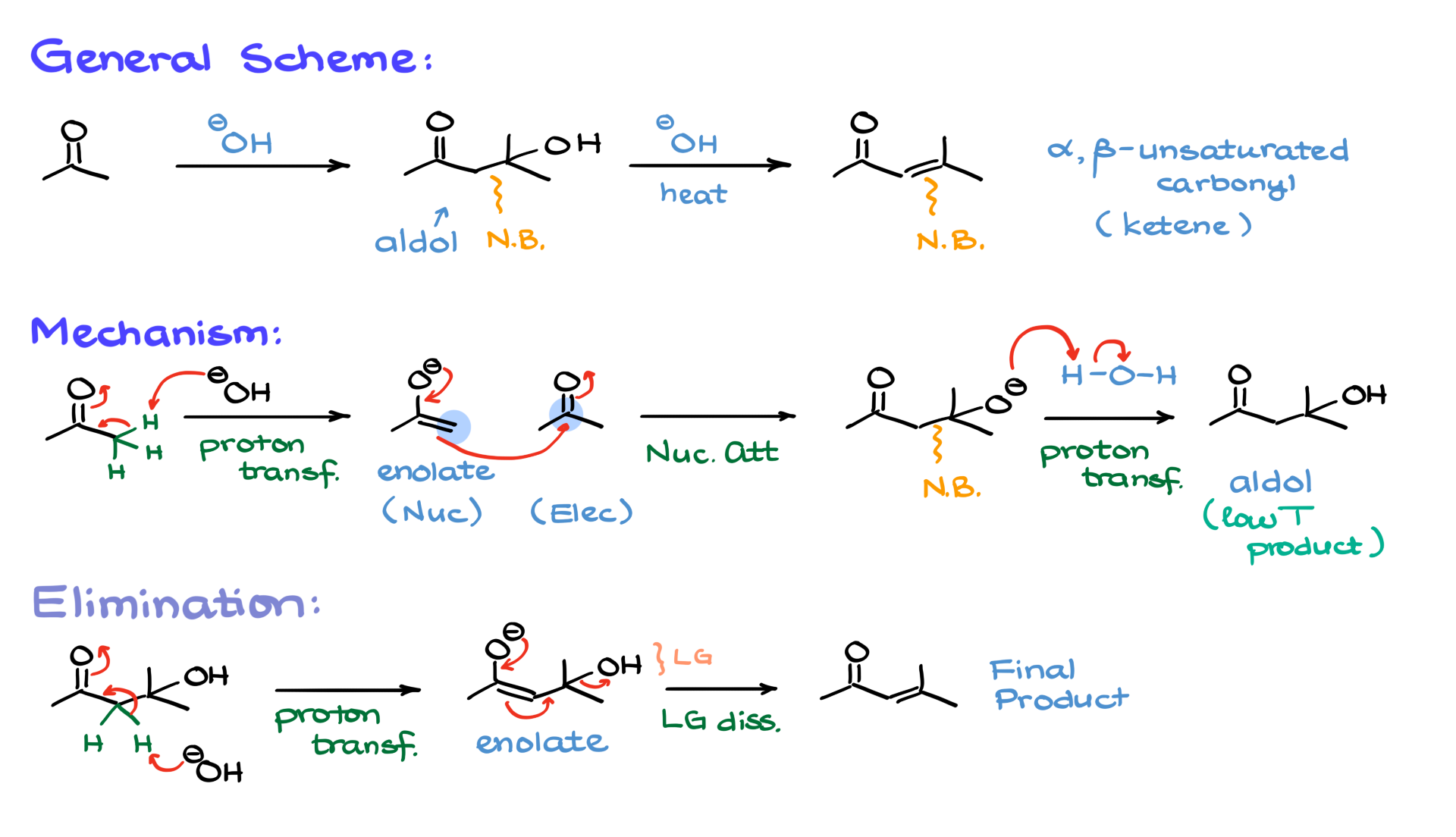
To begin, we take our starting material—acetone in this case—and react it with a base. Typically, this reaction is carried out using sodium or potassium hydroxide. The first step in the mechanism involves a proton transfer, where the base deprotonates the α-position of the carbonyl, generating the corresponding enolate species, which serves as our nucleophile. Since sodium or potassium hydroxide is not a particularly strong base, enolate formation is not complete. The equilibrium constant for this reaction is relatively low—somewhere around 10⁻¹ to 10⁻³—meaning we will have only a small amount of enolate present in solution, while most of the carbonyl remains unreacted.
Since carbonyl compounds are naturally electrophilic, the enolate nucleophile will react with another molecule of the carbonyl compound. This nucleophilic attack creates a new carbon-carbon bond between the α-carbon of the enolate and the carbonyl carbon of another acetone molecule. As a result, we form an intermediate that is negatively charged at the oxygen. This intermediate is then protonated by water in the solution, leading to the formation of the aldol intermediate.
If the reaction is carried out at low temperatures, the aldol intermediate remains the major product, and just like in the acidic version of this reaction, we refer to it as an aldol addition rather than aldol condensation. However, if we want to form the double bond, we need to raise the temperature.
To keep the mechanism clear, I’ll separate the elimination portion. Starting from the aldol intermediate, the next step involves bringing back our base to perform another proton transfer, re-enolizing the molecule to generate an enolate intermediate. From here, the enolate facilitates the elimination step, kicking out the leaving group, which in this case is the hydroxide (OH⁻), ultimately forming the α,β-unsaturated carbonyl compound.
Now, you might be thinking, “Wait a minute, aren’t we always told that hydroxide is a terrible leaving group?” And you’d be right! Under normal conditions, OH⁻ is a strong base and a poor leaving group, especially in neutral or acidic conditions where it would be the most nucleophilic species in the system. However, in a basic environment, we already have hydroxide floating around, so if we push OH⁻ out of our molecule, we aren’t generating anything significantly more basic or reactive than what’s already in the solution. This is one of the few cases where OH⁻ can act as a leaving group, though it’s rare. In fact, this might be the only reaction in your course where you see pure OH⁻ acting as a leaving group. Just keep in mind that in this specific mechanism, OH⁻ can leave.
Now, let’s quickly review the entire mechanism again.
- The reaction begins with a proton transfer, generating an enolate.
- This enolate then attacks another carbonyl molecule, forming a new carbon-carbon bond.
- The intermediate is then protonated, giving us the aldol product. If the reaction is carried out at low temperatures, this aldol remains the final product, and we call it aldol addition.
- However, at elevated temperatures, the aldol undergoes enolate formation again, and from that enolate, elimination occurs, leading to the α,β-unsaturated product.
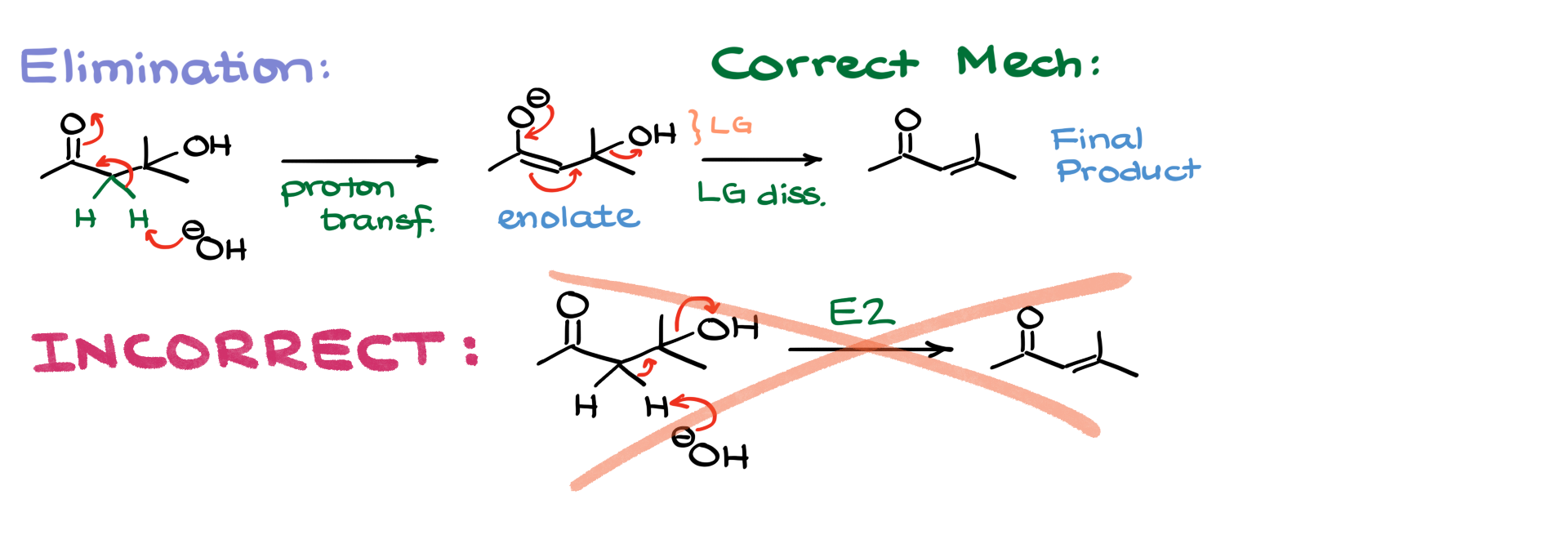
Elimination Warning
Just like with aldol condensation in acidic conditions, I want to emphasize that the elimination step must proceed through enolate formation. It might be tempting to write this reaction as a direct E2 elimination, but that would be incorrect. The enolate must form first, and only then does elimination occur. Always draw your enolate formation before showing the loss of the leaving group.
Mixed-Media Approach
Before wrapping up, I want to introduce an interesting variation that sometimes appears in aldol condensation: a mixed media approach. Don’t confuse it with mixed aldol condensation, it’s not the same thing! We’ll talk about the mixed aldol condensation in a different tutorial.
In some cases, the reaction starts under basic conditions to form the aldol intermediate, but then the medium is switched to acidic conditions for the elimination step. This means that part of the mechanism follows the base-catalyzed pathway up to the aldol intermediate, and then we transition into an acid-catalyzed elimination.
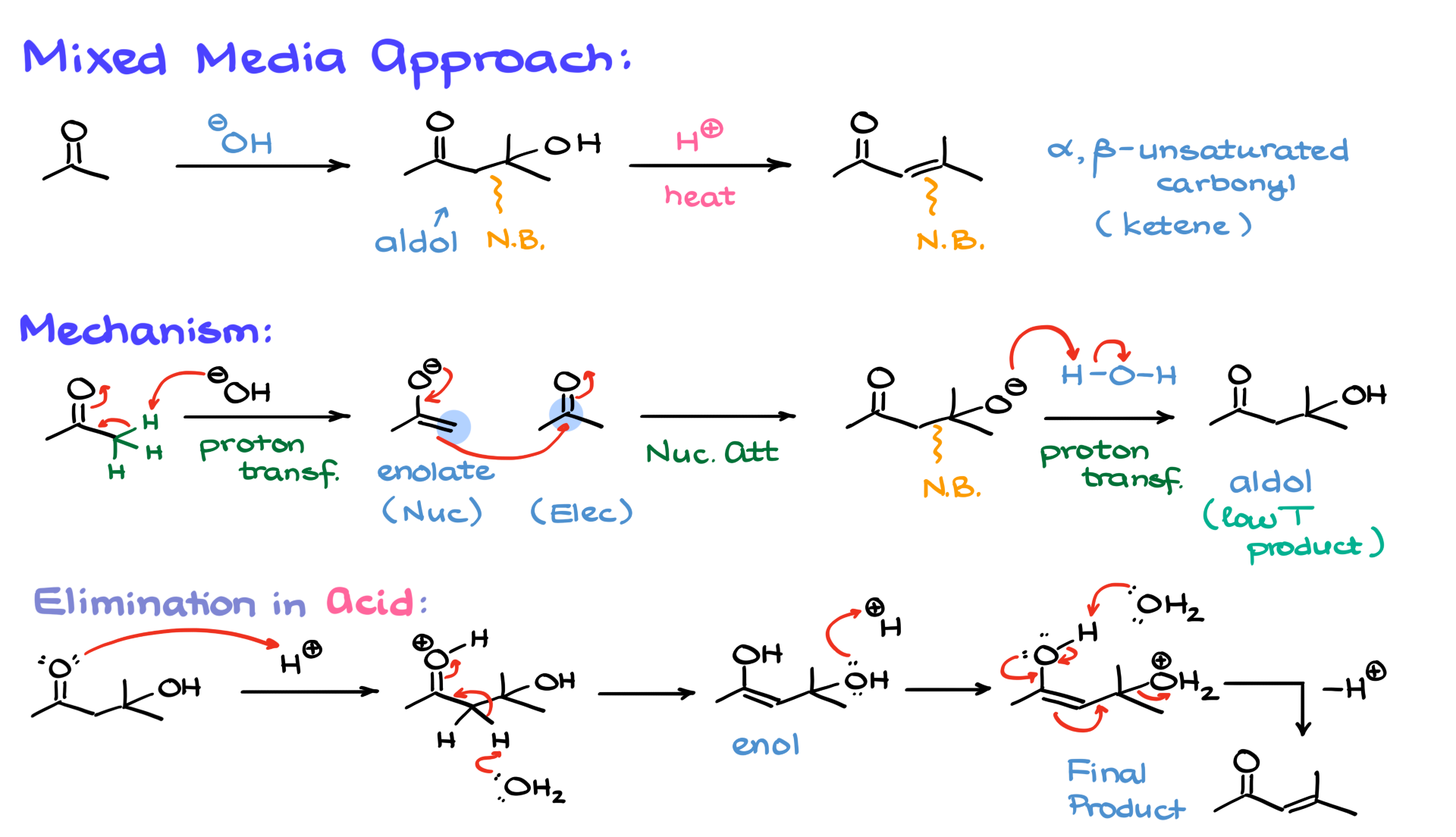
Here’s how that works: Once we obtain the aldol product under basic conditions, we switch to an acidic medium. The carbonyl is protonated, generating an intermediate. A proton is then removed, most likely by water in the solution, forming an enol intermediate. This enol is subsequently protonated at the hydroxyl group, making it a good leaving group. Finally, the enol facilitates elimination, producing the α,β-unsaturated compound as the final product.
So, aldol condensation can proceed in purely acidic media, purely basic media, or through this mixed media approach where the reaction starts under basic conditions and finishes under acidic conditions. Which method is better? Well, that depends. For simple molecules, it doesn’t really matter. However, for more complex molecules with different functional groups, the choice depends on whether the molecule is more stable in acidic or basic conditions. There isn’t a one-size-fits-all answer, but all three approaches are commonly used, so it’s important to understand them. Your instructor will likely introduce all three, and you should be comfortable with their mechanisms, as you’ll probably need to demonstrate at least one of them on an exam.
