Alcohol Synthesis: From Simple to Complex
In this tutorial, I want to show you an easy synthetic strategy to convert simple alcohols into more complex structures. As a prerequisite, you’ll need familiarity with the Grignard reaction, synthesizing alcohols using the Grignard reaction, and the oxidation of alcohols. If you need a refresher, make sure to review those concepts before proceeding.
Assuming you are comfortable with these reactions, let’s dive into our first example.
Example 1: Converting Butanol to a Complex Alcohol
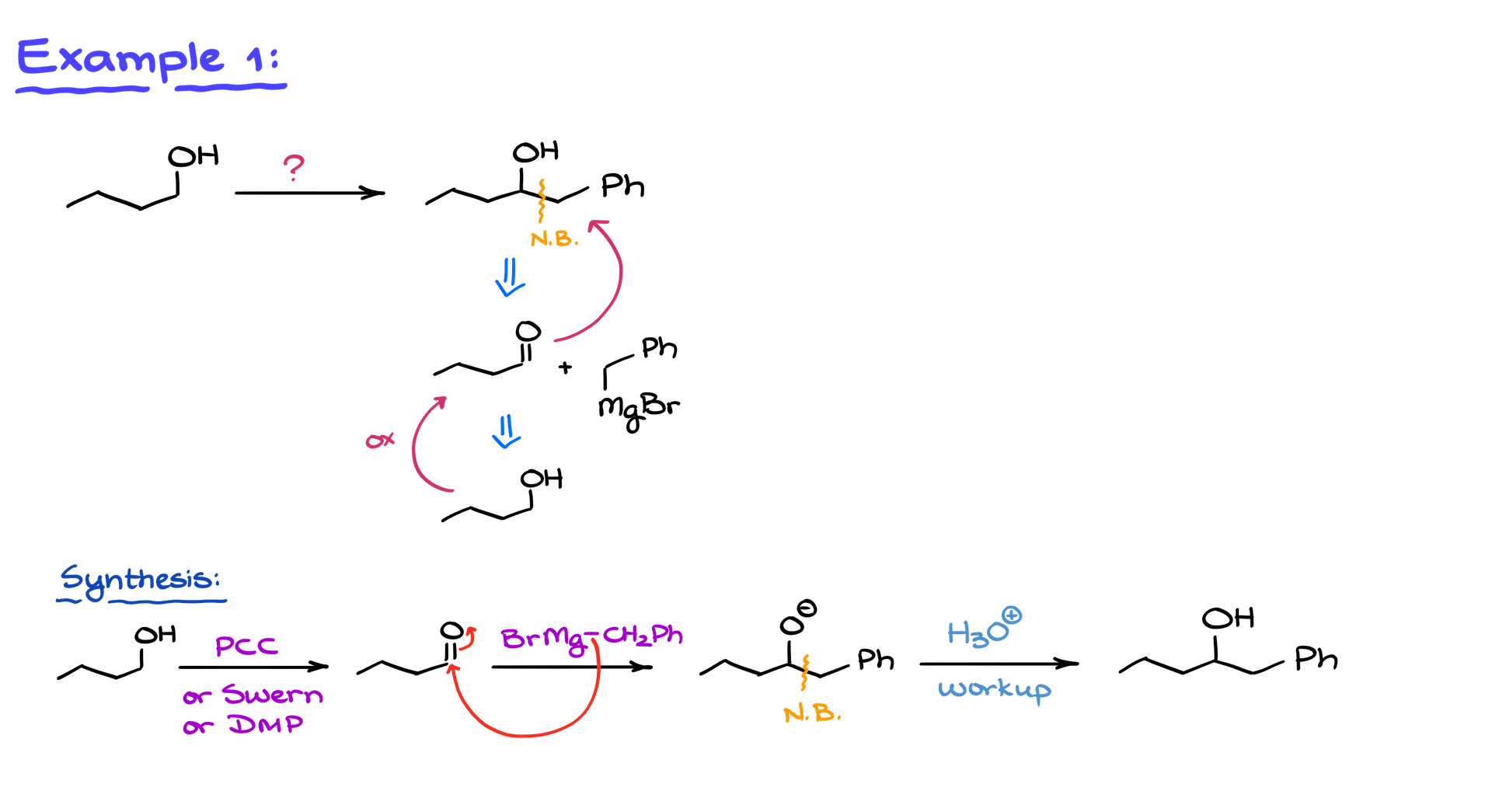
We start with butanol and aim to convert it into a more complex alcohol. Upon analyzing the target structure, we notice a new chemical bond between the original alcohol segment and an added benzyl group. Since this is a carbon-carbon bond, the Grignard reaction is our primary choice, as it is the premier method for carbon-carbon bond formation.
Retro-synthetic Analysis
Thinking about this retro-synthetically, the precursors to our product are an aldehyde and benzylmagnesium bromide (the Grignard reagent). Remember, maintaining the correct carbon count throughout the transformation is critical. Our extra carbon comes from the Grignard reagent, so it’s essential to ensure we don’t lose track of our carbons. Numbering them can be helpful for clarity.
Oxidation Step
To reach the aldehyde from butanol, we perform an oxidation reaction. Using reagents like PCC, Swern oxidation, or Dess-Martin periodinane ensures we stop at the aldehyde and avoid over-oxidation to a carboxylic acid.
Grignard Reaction
Once the aldehyde is formed, we proceed with the Grignard reaction. The benzylmagnesium bromide reagent attacks the carbonyl group, creating an alkoxide intermediate. We then protonate this intermediate with an acidic workup, forming our target alcohol.
Example 2: Adding Two Different Substituents
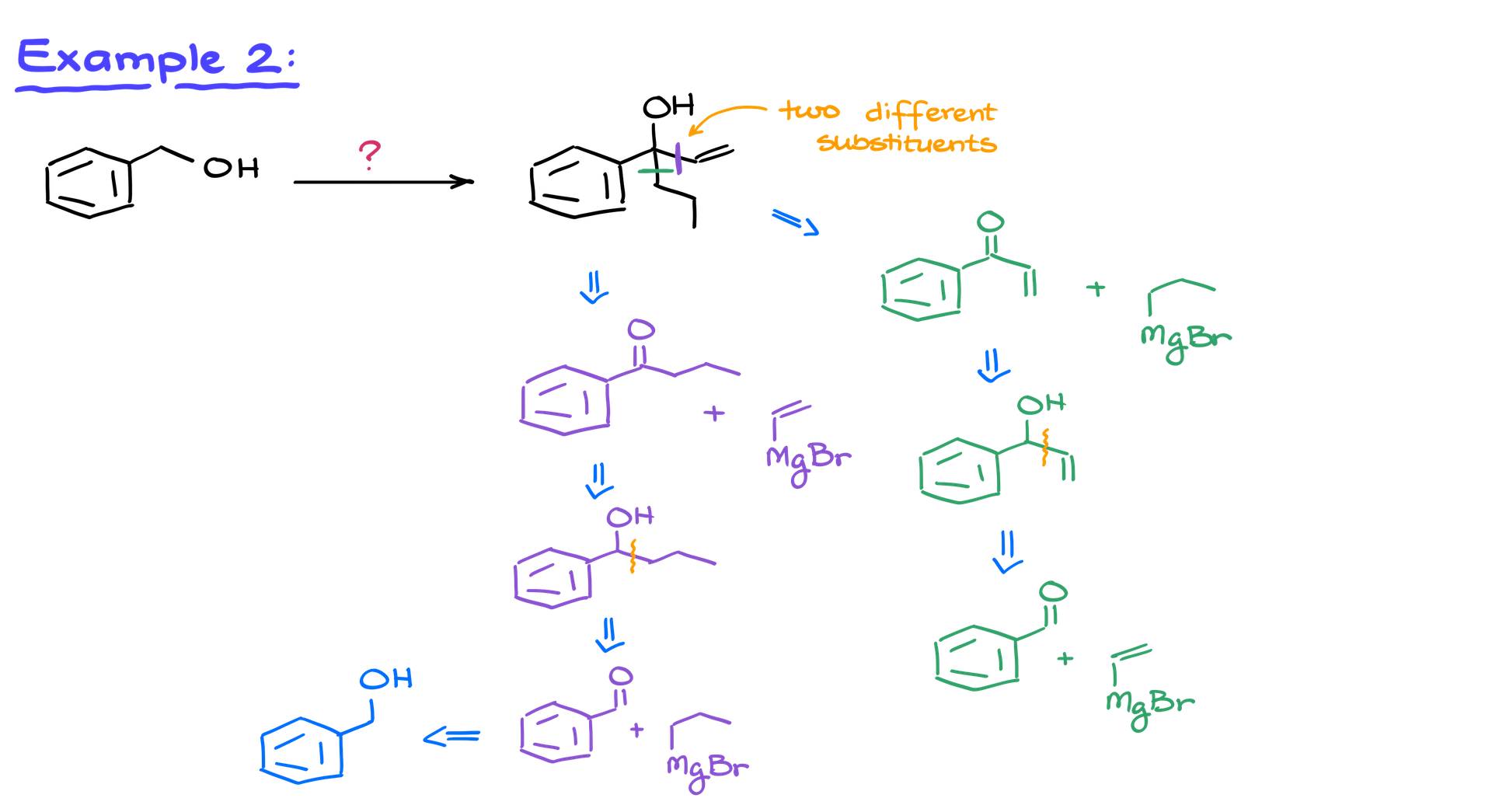
In a more complex example, we need to form two separate carbon-carbon bonds. This involves repeating the oxidation and Grignard reaction sequence twice. Since we’re adding two different substituents, we have options for the order of reactions.
Two Pathways: Purple and Green
1. Purple Pathway: We form a bond with the vinyl group first. Starting from benzyl alcohol, we oxidize it to benzaldehyde. Next, we use propylmagnesium bromide in a Grignard reaction, followed by oxidation to create a ketone. A second Grignard reaction completes the synthesis.
2. Green Pathway: We follow the same initial steps but reverse the order of the Grignard additions. We still oxidize benzyl alcohol to benzaldehyde, but in this pathway, we switch the order of reagents to achieve the final product.
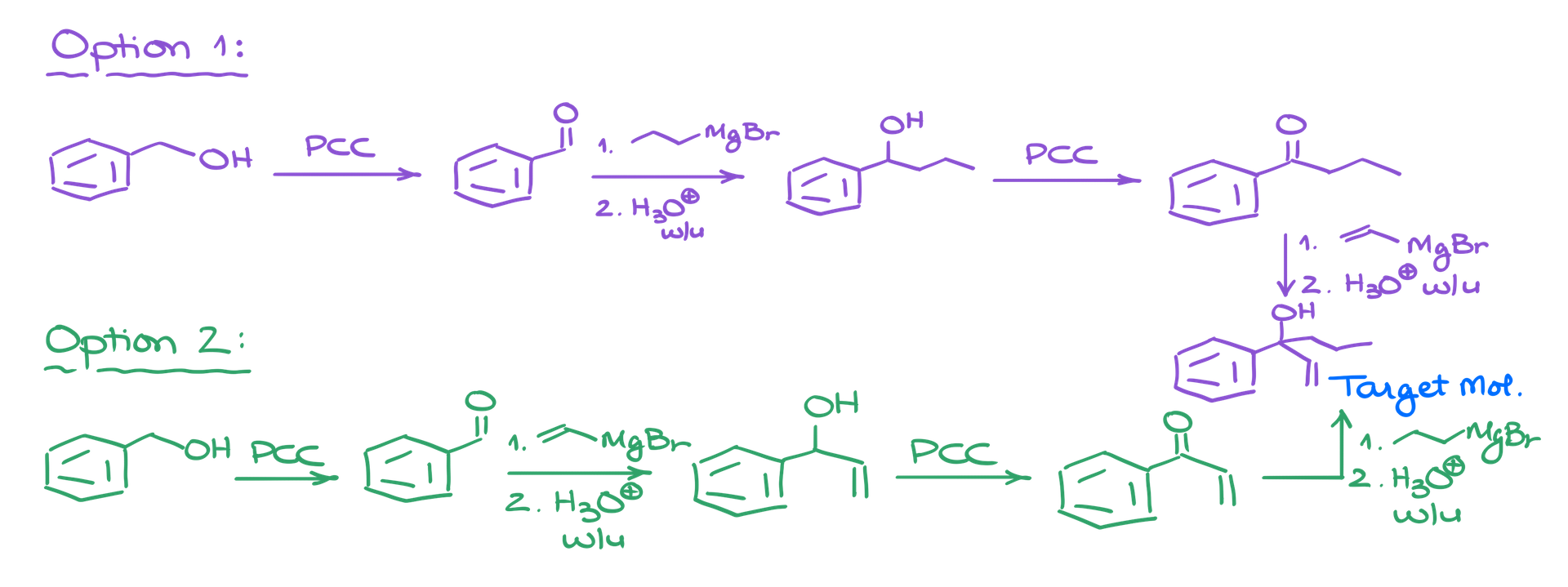
Comparing Both Options
Both pathways yield the same final product. In the context of an introductory organic chemistry course, either method is valid. While there are minor differences in reactivity and yields, these details are not critical for our purposes here.
Example 3: Secondary Alcohols and Cyclopentanone
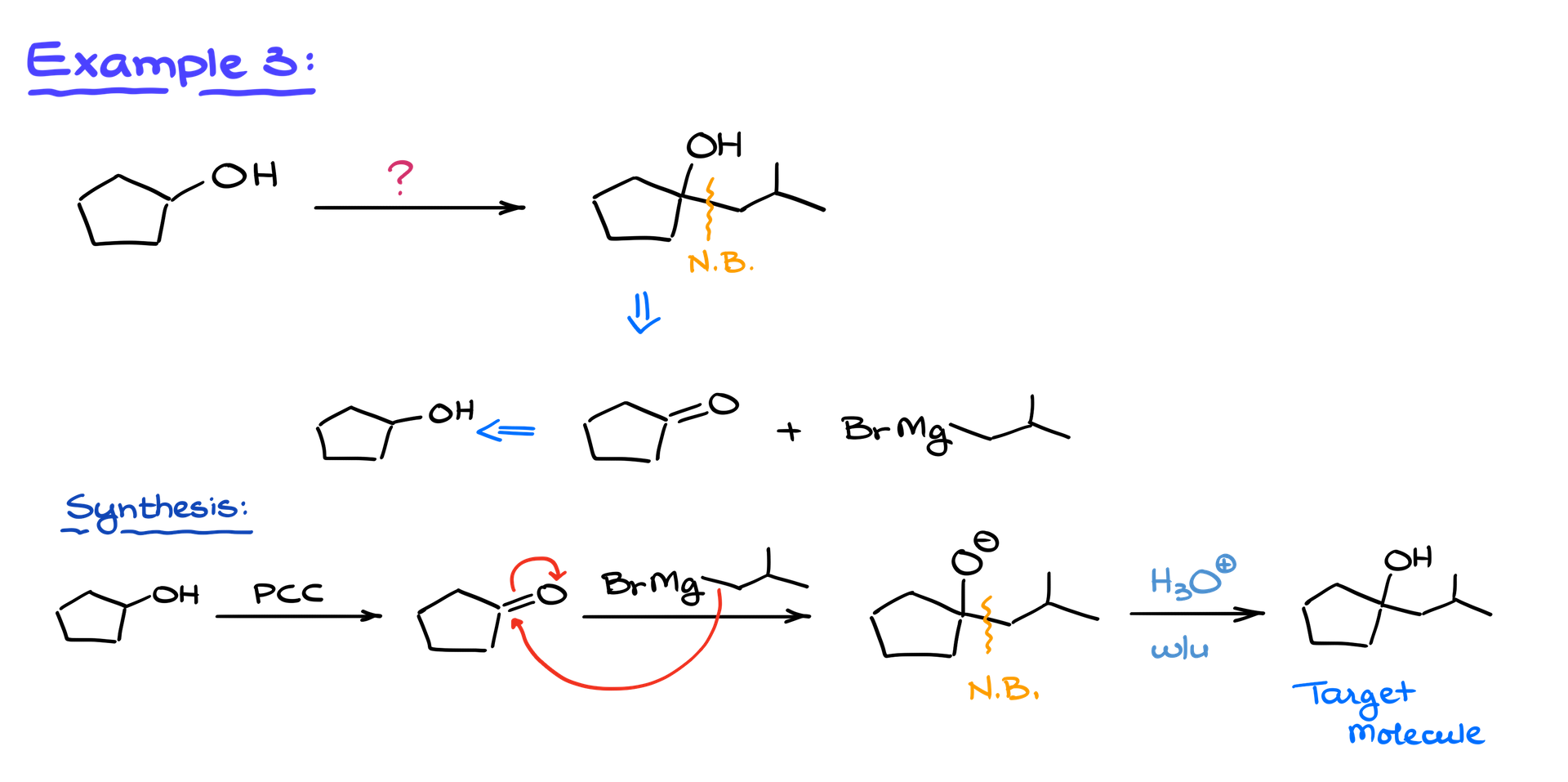
Let’s consider a final example starting with a secondary alcohol. We need to form a new carbon-carbon bond. Using our Grignard retro-synthetic approach, we identify cyclopentanone as an intermediate, which comes from cyclopentanol.
Oxidation and Grignard Reaction
We oxidize cyclopentanol to cyclopentanone using PCC or a similar oxidizing agent. Since it’s a secondary alcohol, there’s no risk of over-oxidation, so you have flexibility in choosing your reagent. The Grignard reagent then attacks the carbonyl, forming an alkoxide intermediate. A final acidic workup protonates the alkoxide, yielding the target alcohol.
Key Takeaways

Although these syntheses may seem complex, they rely on repeating the same steps: oxidation followed by a Grignard reaction. The general pattern is to:
1. Oxidize the alcohol to a carbonyl compound.
2. Perform a Grignard reaction to create a more complex alcohol.
3. Repeat as needed, depending on the desired complexity.
For example, transforming a primary alcohol into a tertiary alcohol involves passing through an aldehyde and a secondary alcohol before reaching the final product. You can start anywhere in this sequence and work up to your desired structure.
By understanding and following this systematic approach, you’ll be able to tackle alcohol-to-complex-alcohol syntheses with confidence.
