Alcohol Protecting Groups
Alcohol protecting groups are a pretty big deal in organic chemistry. Well, many topics are important. Why should you care about the protecting groups specifically?
Well, alcohol functional group is very versatile in terms of its reactivity and chemical transformations it can participate in. And precisely this high chemical reactivity is often a problem when we’re trying to perform some sort of chemistry and an alcohol functional group interferes with it.
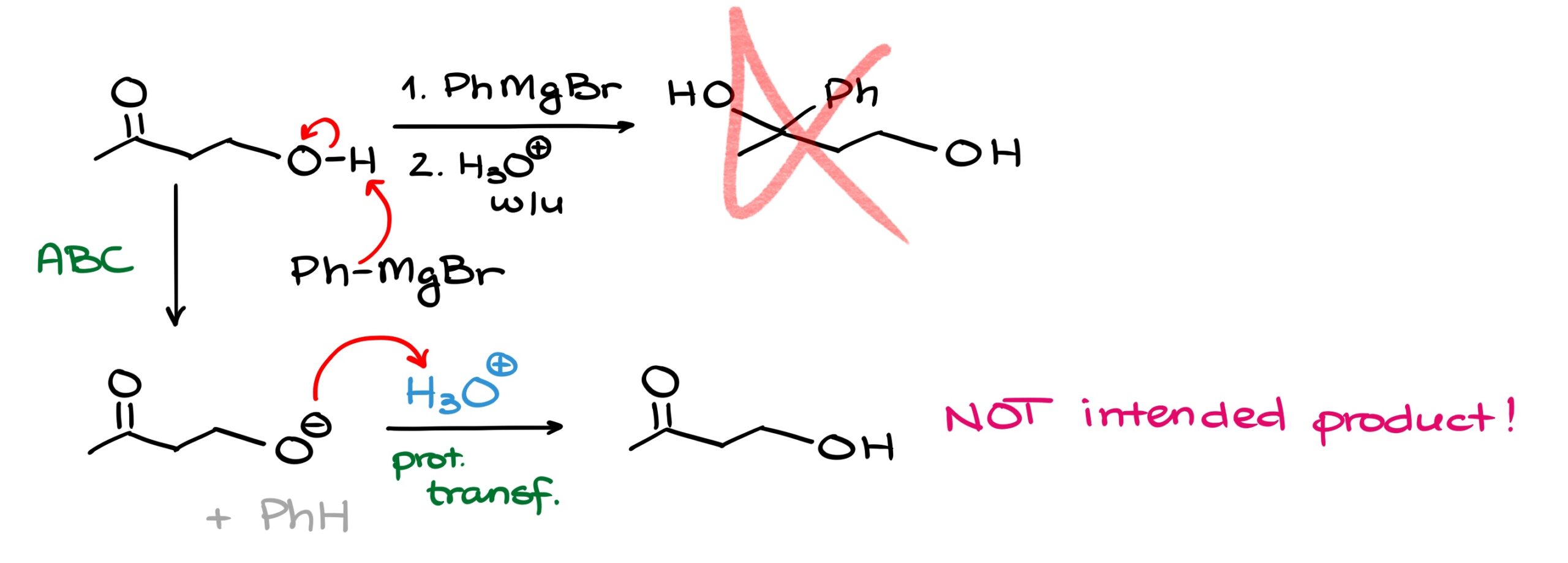
For instance, if I tried to do a Grignard reaction with this molecule, I would face a problem. And the problem here is that the Grignard reagent, phenyl magnesium bromide in this case, is not only an excellent nucleophile, but also a strong base. So, instead of attacking the carbonyl, we’re going to have the acid-base interaction leading to the formation of the alkoxide.
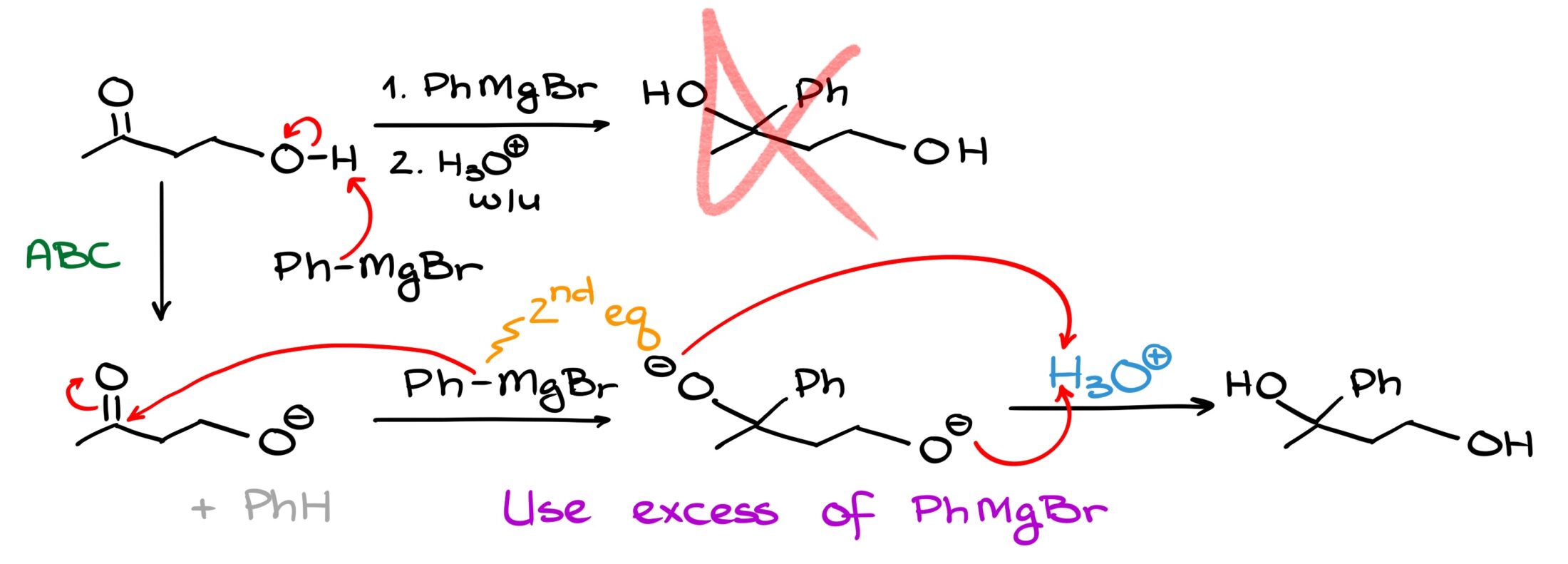
You might argue that this is not that big of a deal, we’ll just use two equivalents of our Grignard reagent and get what we want in the end. And you would be right in this particular case. We can certainly do that here. But that’s only possible because the Grignard reagent we’re using is dirt cheap and easy to make. This approach would be a horrible waste of time and material if, say, instead of something simple like phenyl magnesium bromide, I used a complex reagent that I spent days or maybe even weeks making.
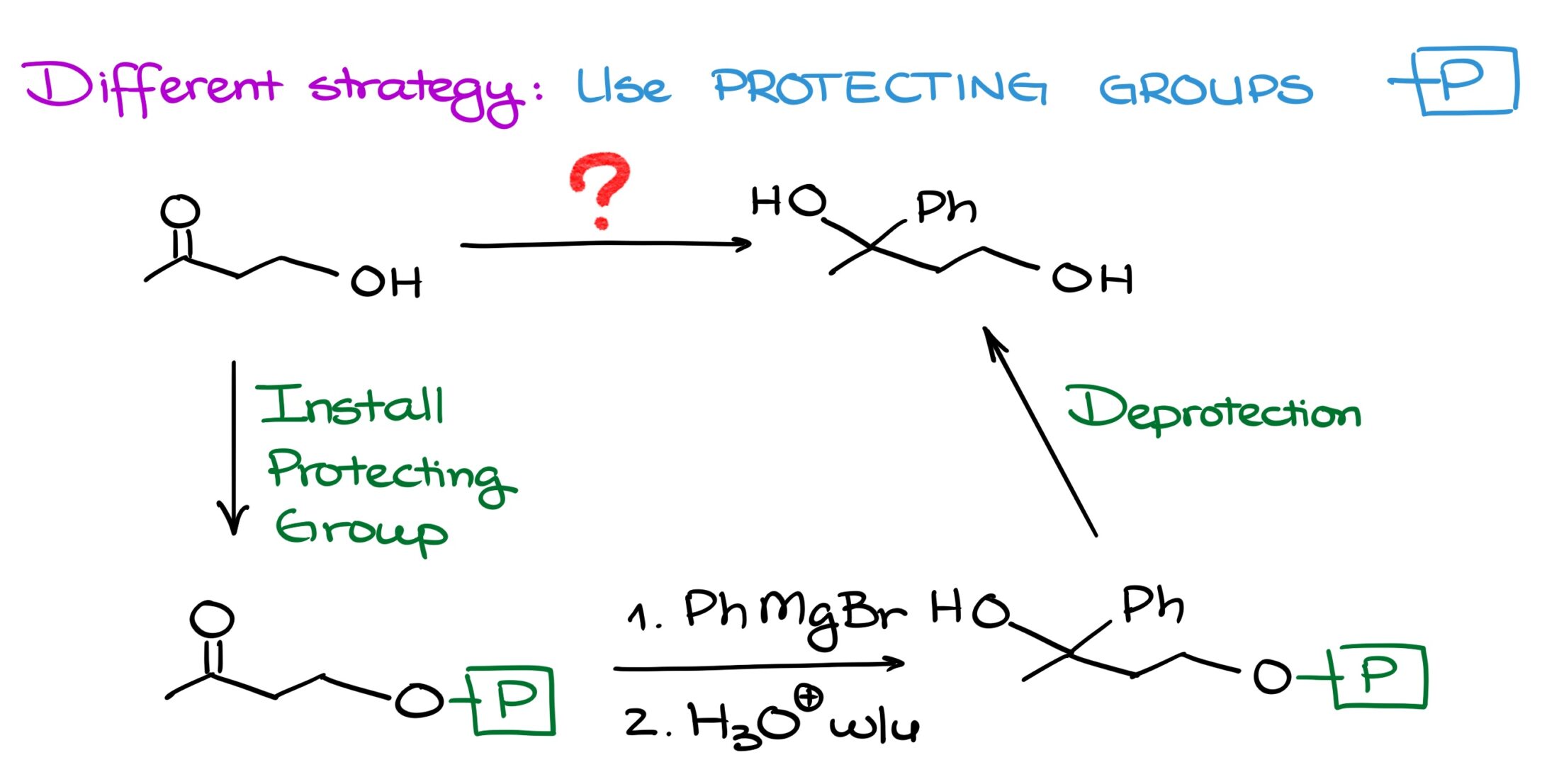
This is where protecting groups come into play. You see, instead of using two equivalents of our Grignard reagent, we can place a temporary protecting group onto our alcohol. This way, alcohol won’t be able to mess up our reaction. And once we’re done, we can take the protection off regenerating the original functional group.
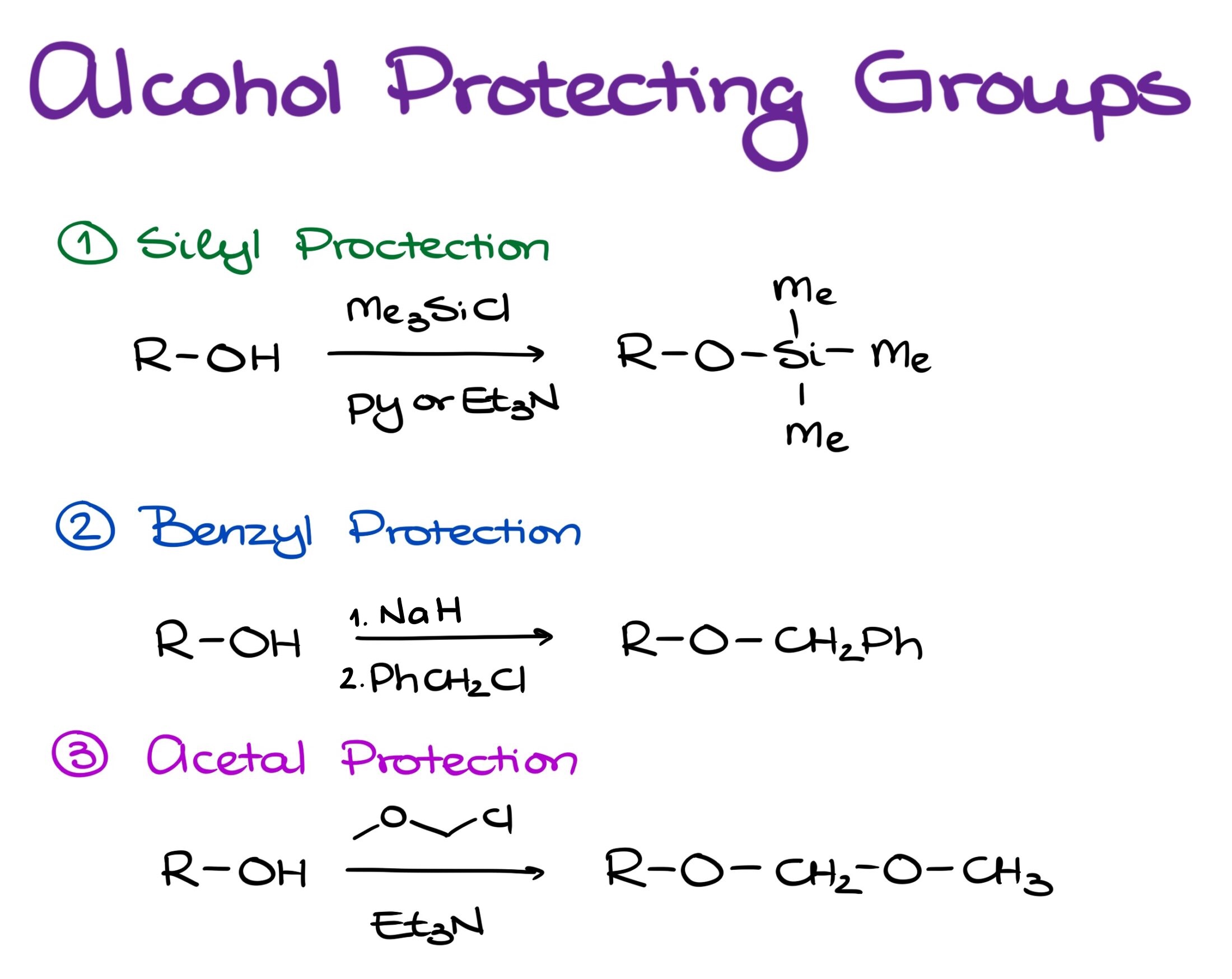
There are three common types of protecting groups that we use for alcohols:
- Silyl protection
- Benzyl protection
- Acetal protection
Each of these protecting groups has its strengths and weaknesses. The most universal of all these three is the silyl protection, so let’s look at it first.
Silyl Ether Protecting Groups

The two most common silyl ethers we are going to convert our alcohols into are:
- TriMethylSilyl ether (TMS)
- tert-ButylDiMethylSilyl ether (TBDMS)
We use the second one when we typically want the alcohol to turn into a more bulky group for finer steric control of our reaction. It also seems to be a bit more resilient to various reaction conditions. For our purposes, however, these are interchangeable, so I’m going to use the TMS as an example structure here in my reactions.
Applying the Protecting Group
First, how are we going to apply our protecting group onto our molecule? In the case of the silanes, we’re going to use the corresponding chloride. So, if I wanted to add a TMS to my molecule, I would use the corresponding trimethylsilane chloride in pyridine as a solvent.
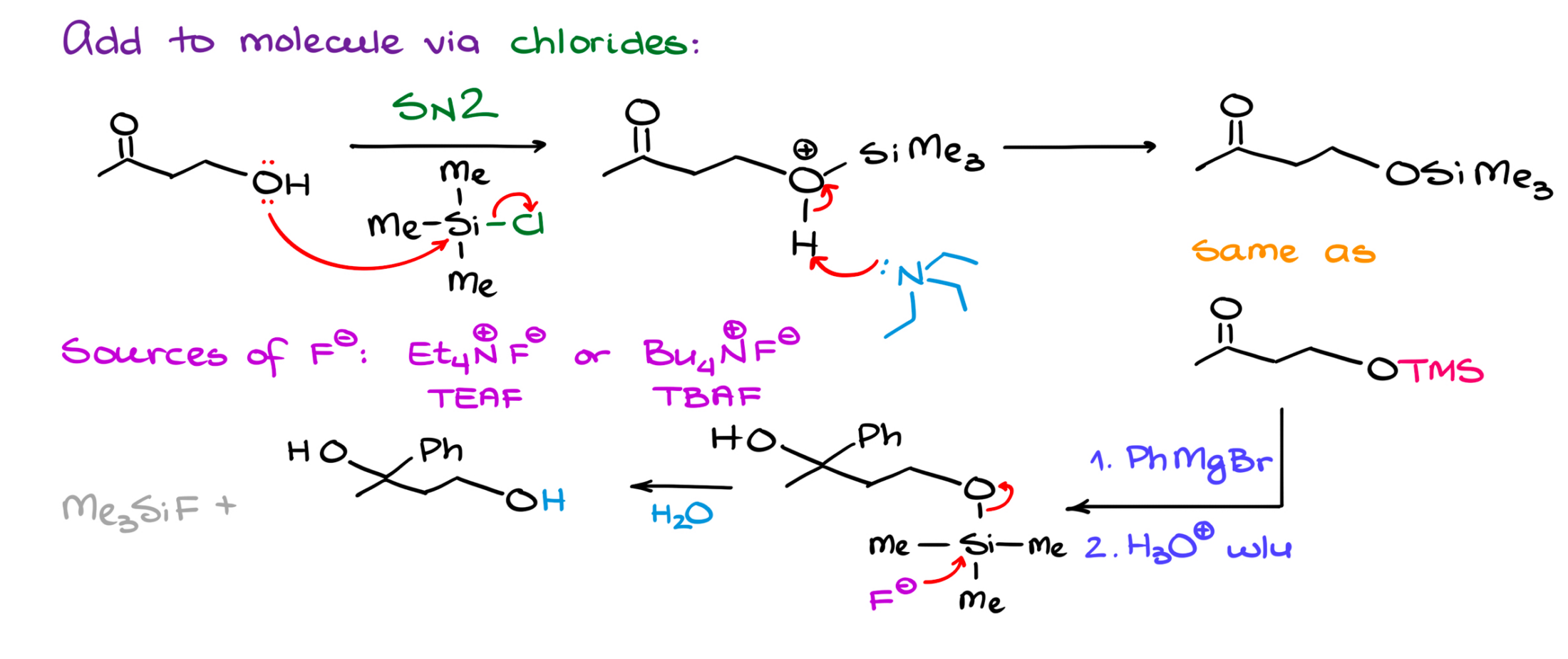
The mechanism here is very straightforward SN2-style substitution. While our alcohol is not necessarily a very good nucleophile, silane here is rather electrophilic. So, it easily reacts with the alcohol. And after pyridine deprotonates our intermediate, we get the final product with the protecting group sitting on our molecule.
Of course, if you have a few nucleophilic centers in your molecule, you might find yourself in a situation where you’ll have silicon sitting in multiple places in your molecule. So, when you are planning your synthesis, it’s always a good idea to introduce your protecting group at an appropriate point where it’s not going to jump on multiple atoms making a mess. It’s hardly going to be an issue with simpler molecules, but can certainly muddle the waters with more complex substrates.
So now, when I have my alcohol protected, I can easily do my reaction and not worry about alcohol messing things up!
Removing the Protecting Group
Cool, we’ve done our chemistry, everything worked the way we anticipated, now what? We still have that protecting group sitting on our molecule. So, we need to get rid of it somehow.
As I’ve mentioned, silyl protection is incredible resilient to anything. This means that it’s going to stay put in both acidic and basic conditions. So, does it mean we’re stuck with it forever? No, not really. While the silyl protection might be very resilient, it has one major weakness. And this weakness is the fluoride ion.
If we treat our product with anything containing fluoride, it will react with silicon and displace it via a simple SN2 reaction. The easiest source of a fluoride is hydrofluoric acid, HF. However, since the glassware we use in the lab is silicon-based, HF tends to dissolve the glass just as easily as taking the silyl ethers off our molecule. So, unless you have platinum beakers (and btw, yes, they do exist) in your lab, you’re out of luck and need another source of fluoride.
Ionic fluorides like KF or something similar have one major problem—they don’t dissolve in organic solvent. So, we need something more creative to kill both birds with the same stone—bring fluoride to our molecule and have it in an organic-solvable form.
And the solution here is quite elegant. To make our ionic fluoride solvable in organic solvents, we’re going to use organic cations as our counter ions. The two most common reagents are tetraethylammonium fluoride (TEAF) and tetrabutylammonium fluoride (TBAF). These reagents are quite soluble in organic solvents, and they drag the fluoride ion with them into the solution delivering it to where we need it.
Alcohol Protection via Acetals
As I’ve mentioned at the beginning of this tutorial, silyl protection is one of the three main protecting groups we typically see for alcohols. The next method we’re going to see sometimes is the use of acetals. Typically, we’re going to see either a dihydropyran (DHP) or methoxymethyl (MOM) protecting groups in this segment.
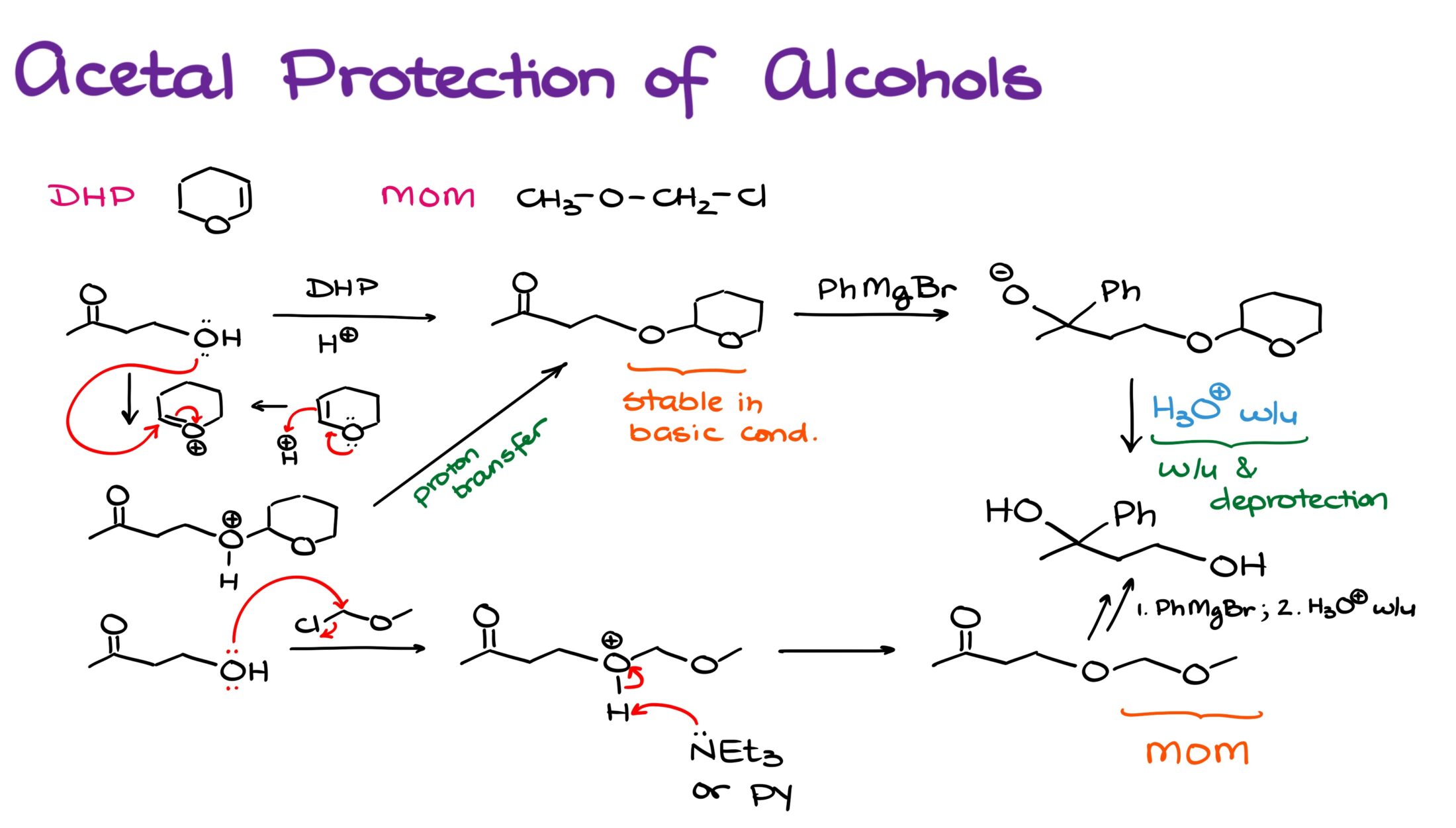
We add the DHP group onto the alcohol in acidic conditions. This is a typical electrophilic addition to an alkene reaction. In this case, the alkene is especially susceptible to the reaction due to the polarization from the oxygen atom.
Once the protecting group is on, it’s very resilient towards the basic conditions. However, it’s sensitive to acids like any acetal. And will easily pop off in acidic conditions. This is especially convenient since we can often combine deprotection step with the acidic workup that we often see in many reaction in organic chemistry.
Methoxymethyl protecting group, on the other hand, is installed in a similar fashion to the silyl protecting group—basic conditions via an SN2 reaction. And similar to DHP, MOM is sensitive towards acids and will easily pop off in acidic conditions.
I also wanna point out that if you opt to use one of these two protecting groups, you should probably go with DHP unless your substrate is very sensitive towards acids. While MOM is an excellent protecting group, MOMCl is quite toxic and carcinogenic. So, you should avoid this “mom” at all costs.
Benzyl Protecting Group
Finally, we have a benzyl protecting group. This is an incredibly resilient protecting group and we often see it when dealing with glycochemistry, which is chemistry of carbohydrates.
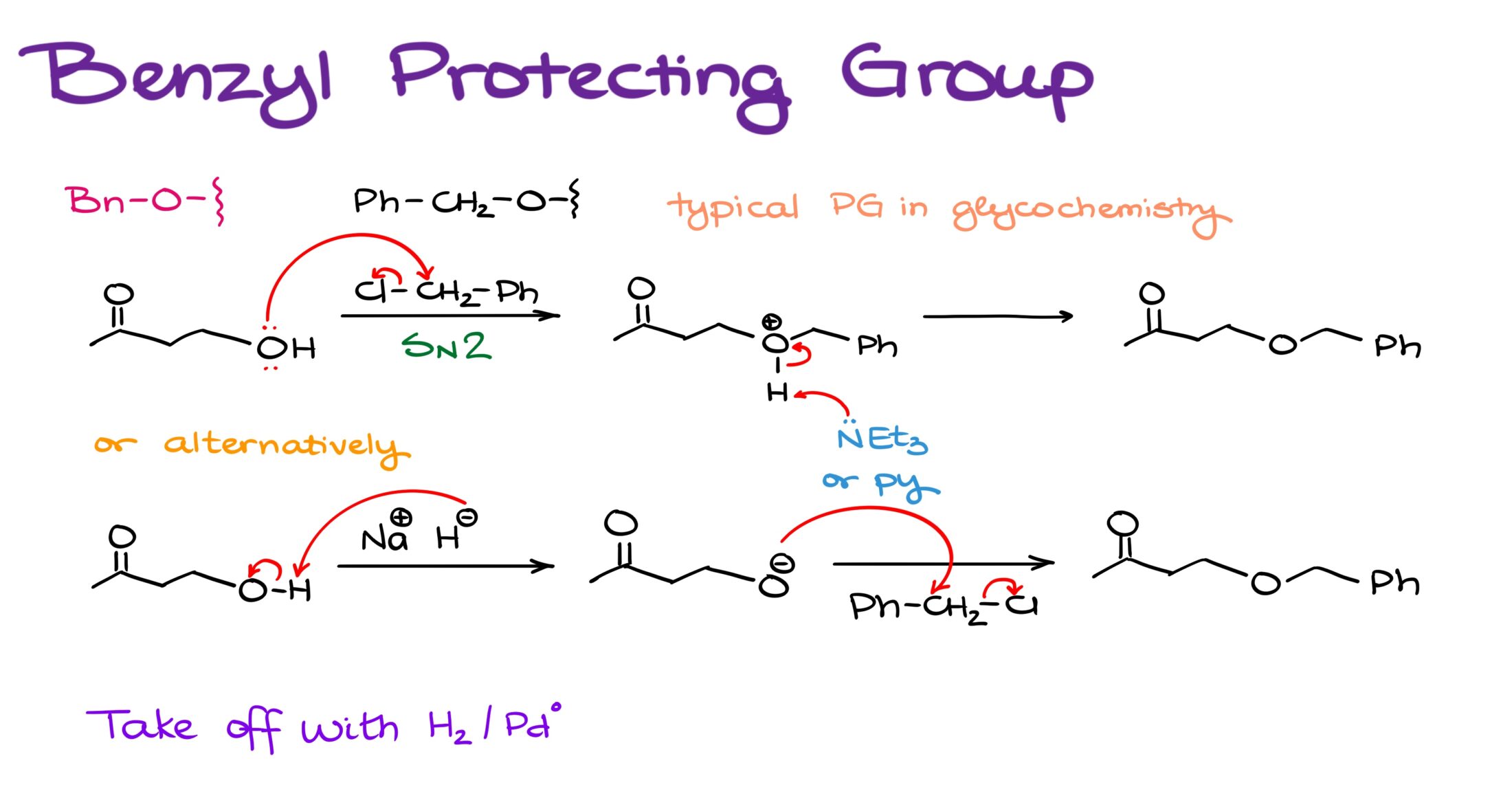
Typically, we install the benzyl protecting group via an SN2 reaction of the corresponding alkoxide and benzyl halide. This is a typical Williamson ether synthesis, so there’s nothing special about this reaction. By now, you should’ve already seen this reaction in your course.
Although, we didn’t look at it from the perspective of its utility as a reaction that makes a protecting group.
What’s important about this protecting group is that it’s bulky and chemically resilient. However, we can easily take it off using hydrogen on palladium catalyst. Generally, we use this type of chemistry when we want to reduce a double or a triple bond. However, benzylic position, just like Albus Dumbledore, has its own privileges. And if you have a C-O bond in benzylic position, it can be broken by a simple reaction with hydrogen over heterogeneous catalyst. It turns this deprotection into the cleanest reaction out of all deprotections that we have seen here so far. However, it comes with its disadvantages as well. Namely, if you have double or triple bonds in your molecule, you’ll end up reducing them as well in addition to taking your protection off, which is, of course, not ideal.
Concluding Thoughts
So, as you can see, there are options when it comes to the alcohol protecting groups. Most likely, you’re going to be using the silyl protection as your main go-to protecting group for alcohols. However, if you see any of the other ones, you’ll know what they do and why we use them.
As a general rule of thumb, you want to design your synthesis with the least number of steps possible. And using protection adds two steps to your overall scheme: one step to install the protecting group, one step to later remove it.
