Typical Acid-Base Exam and Homework Questions
In this lesson, I want to talk about the most common acid-base questions that show up on an organic chemistry test and how to solve those questions using the pKa table.
Most Common Types of the Acid-Base Questions
Typically, you’re going to see the following questions:
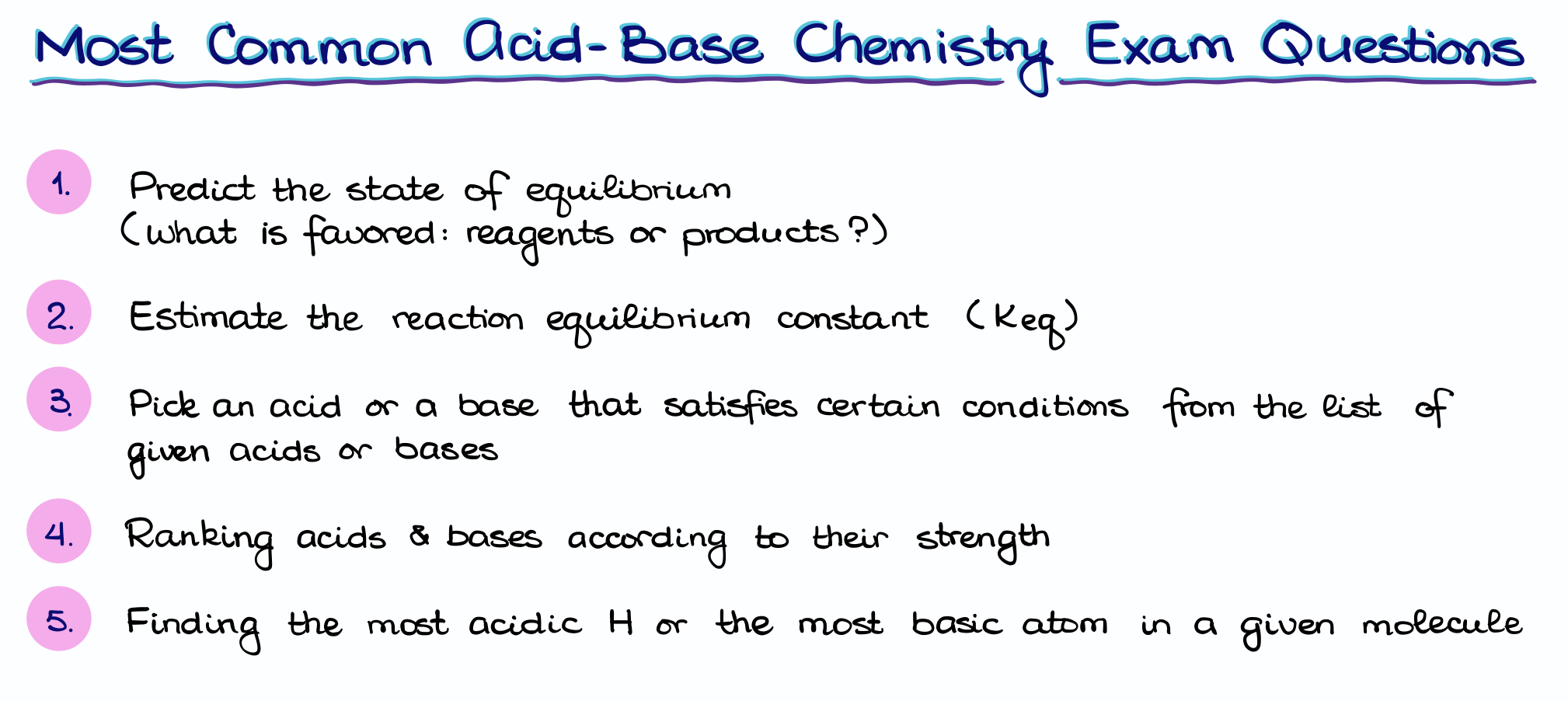
- Predict the state equilibrium in a given reaction.
- Estimate the equilibrium constant for a reaction.
- Pick an acid or a base according to a set of conditions.
- Rank acids and bases according to their strength.
- Finding the most acidic hydrogens or most basic atoms in the molecule.
And, as I’ve mentioned a moment ago, we are going to look at all these types of questions assuming you have the pKa table in front of you. So, go ahead and pull out your table so you can follow along.
Predicting the State of the Acid-Base Equilibrium
So, the first common question is going to revolve around predicting the state of equilibrium. When we are talking about predicting the state of equilibrium, we are asking which side of the equilibrium is favored. Is it going to be the reactants or is it going to be the products in our reaction?
Normally, you’ll have a reaction given to you. And the first thing we want to do is look at it an identify the parts of the reaction.
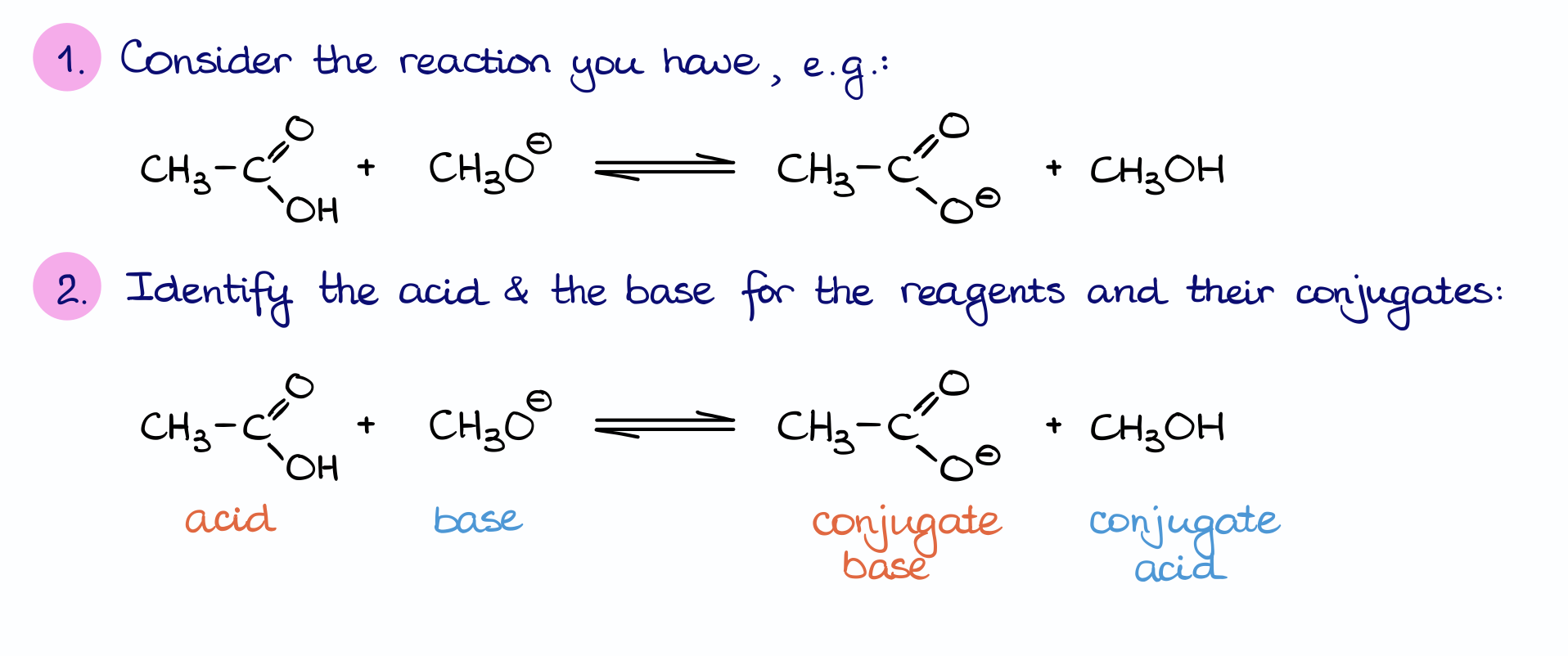
Here’s a simple acid base reaction between acetic acid and methoxide ion. Once we make sure we have the reaction written out, we need to identify the reaction parts: what is the acid and what is the base.
On the reactants side, we have the acid (acetic acid) and a base (methoxide ion). I want to quickly remind you here that we are typically looking at the Brønsted-Lowry definition of acids and bases. And according to the Brønsted definition, the acid is the donor of the proton, while the base is its acceptor. In this case, we can see that the acetic acid molecule lost the proton and methoxide anion gained one.
Now, on the product’s side, we can identify the corresponding conjugates. In this case, the acetate ion will be the conjugate base, while the methanol will be our conjugate acid.
Making a Decision on the State of the Equilibrium
With this information at hand, we have two options.
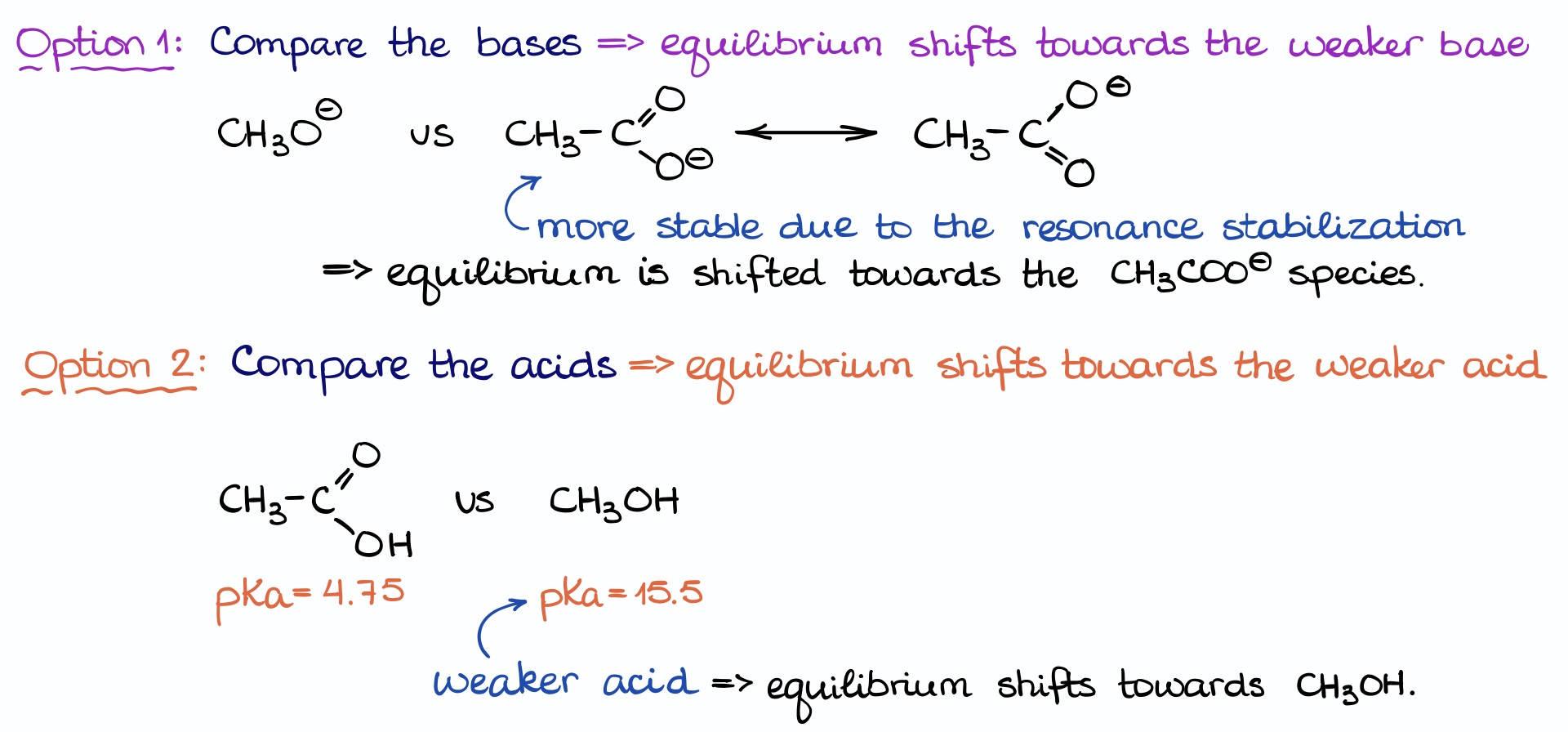
First, we can compare the stability of the basic species on each side. The acetate ion here is more stable due to the resonance stabilization of the charge. And as the equilibrium favors the more stable species, it will favor the formation of the acetate ion. But as we’re looking at the comparisons using the pKa values, this particular method is not what we are going to be using here.
So, we are going to look at option two, where we compare the pKa values for the acids on each side. Using my pKa table, I can see that the acetic acid has a pKa is 4.75, while methanol’s pKa is 15.5. This means that methanol is a much weaker acid than the acetic acid.
Remember, the higher the pKa value, the weaker the acid!
And the equilibrium favors the weaker acid. It’s an especially important thing to keep in mind: in any reaction you are always going to be favoring the weaker species. So, if you’re working on a reaction and suddenly you’ve made a strong acid or a strong base, double check what you’re doing, you may have made an error!
Final Thoughts on the Equilibrium
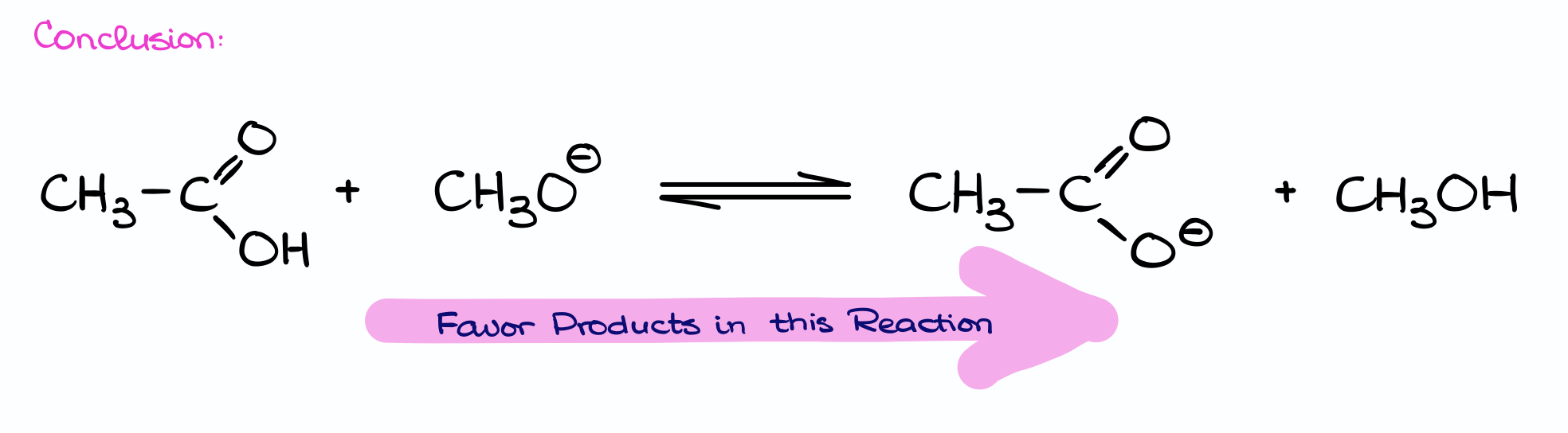
In conclusion, based on the pKa values we get the equilibrium favoring the products in this reaction. Notice, that both “options” agree here: we’re favoring the more stable base (the acetate ion) and the weaker acid (methanol). This is also an important observation because if one factor points you to one direction and another one points you to the other direction, you’re missing something. So, using multiple ways to analyze your reaction is perfectly fine and they all should be pointing you to the same conclusion.
Calculating the Equilibrium Constant for an Acid-Base Reaction
Now, how about calculating the equilibrium constant (Keq) for a reaction? Let’s look at the very same example we’ve worked through a moment ago and calculate the Keq for it.
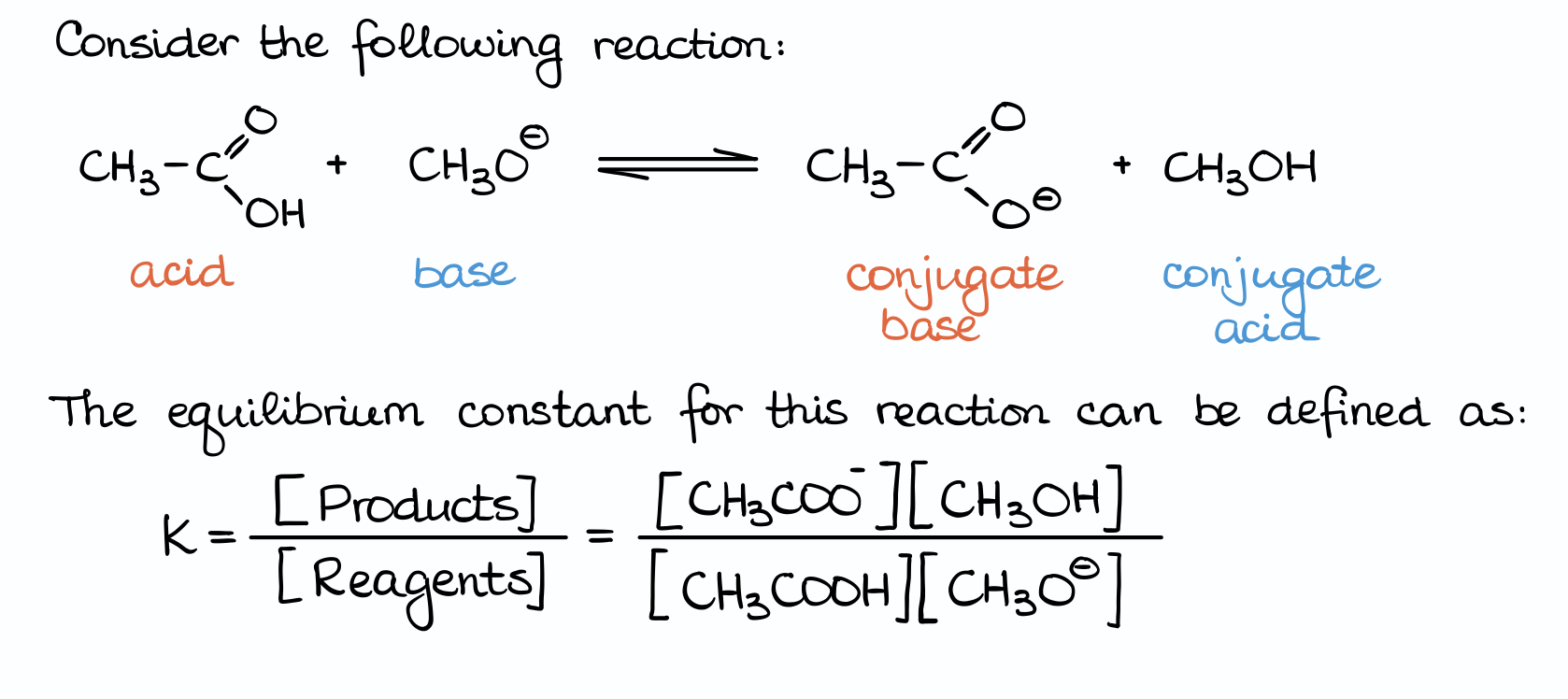
By the definition, the equilibrium constant is the ratio between the concentrations of the products and the reagents in a reaction. And here we have a little bit of a problem because we have no idea what those concentrations are. And I can guarantee, that you’ll never get those on the exam. Well, at least I’ve never seen a question with concentrations in 16 years of me working with students from all over the place.
So, what do we do?
The information that we can get is the pKa values from the pKa table. Can we possibly use those? Let’s see!
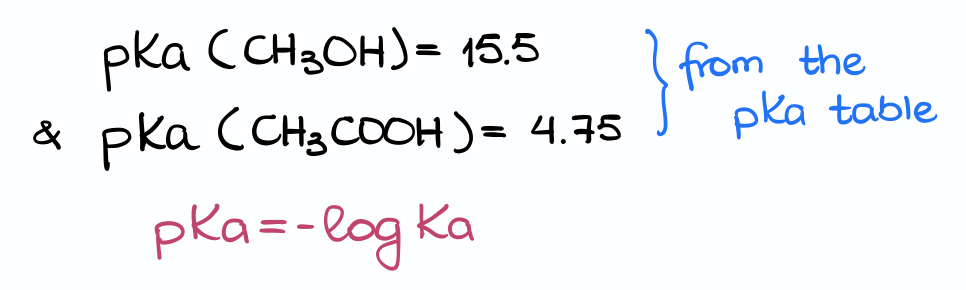
The pKa values for the acetic acid and methanol from my pKa table are 4.75 and 15.5, respectively. We also know that by definition, the pKa = -log(Ka). So, what will I get if I write the expressions for the Ka values for each of my acids here?
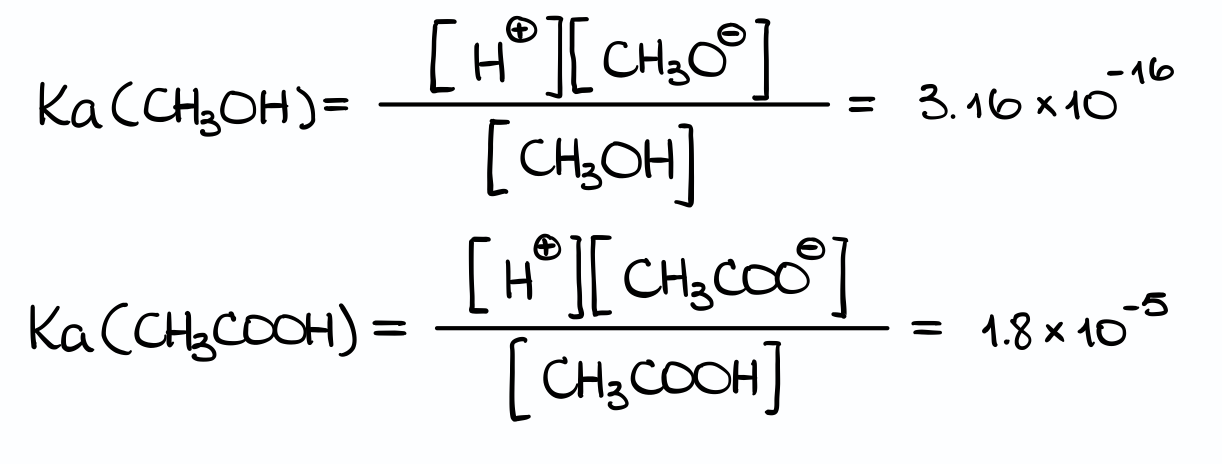
We’ll get something like this. I’ve also back-calculated the Ka values by solving the pKa = -log(Ka) equation for Ka for each of my molecules.
Rearranging the pKa Value Expressions
And now here’s something really cool we can do to our equilibrium constant expression. We can take our expression for the equilibrium constant, multiply nominator and denominator by [H+] to get the following two fractions. You can see that the first fraction is the Ka of the acetic acid, while the second one is the inverse Ka expression for methanol.
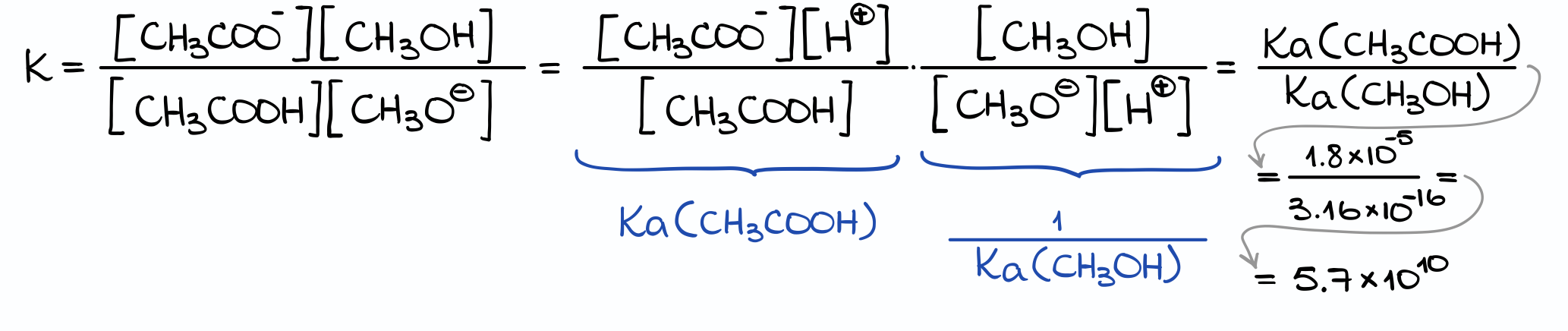
Thus, the Keq for our reaction equals to the ratio of the Ka values for the acids we have in the reaction! So, we can use the tabulated date and don’t actually need any experimental concentration values to calculate the equilibrium constant for a reaction at normal conditions. Very convenient!
Finally, by plugging the corresponding numbers in, I get my Keq value.
This method, however, is a little bit tedious. We normally don’t operate with the pure Ka values, so you’d need to calculate those every single time if you want to do this calculation this way. Which is not ideal. What we can do instead, is to use the shortcut.
pKa to K Shortcut
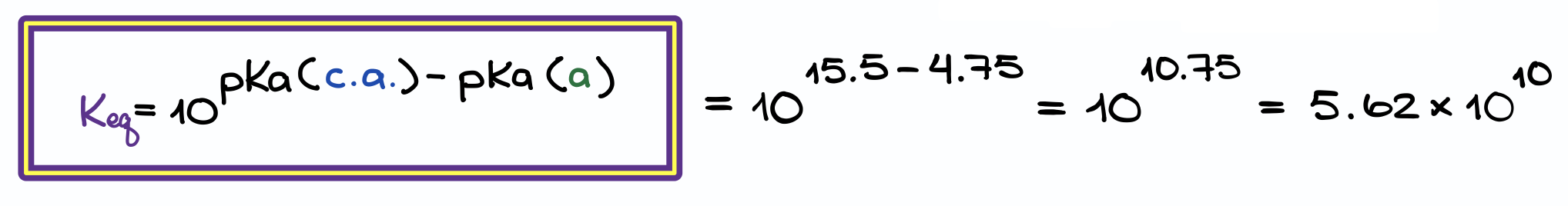
And the shortcut is that the Keq = 10pKa(c.a.) – pKa(a). And as we normally have the pKa values readily available to use, estimating the equilibrium constant becomes a simple algebraic manipulation.
You can see there’s a little discrepancy in my numbers using the two different methods, but it’s simply due to the rounding error between the steps. Within the scope of organic chemistry, it’s never going to be an issue. In organic chemistry we are generally concerned with the ballpark estimation rather than precise calculations.
In many cases, your instructor may be even fine with not converting your numbers into a proper scientific form and just leave is as 10 to some odd power. Like what we have here—1010.75 is going to be quite alright with the majority of instructors.
Choosing the Acid or a Base from the List According to Some Criterion
Ok, we’ve seen how we can use the pKa values to get the equilibrium and calculate the equilibrium constant. What else can you expect on the test?
Some instructors LOVE questions when you have to choose an acid or a base to use in a reaction based on some sort of criteria.
Example 1
Let me illustrate this with an example:
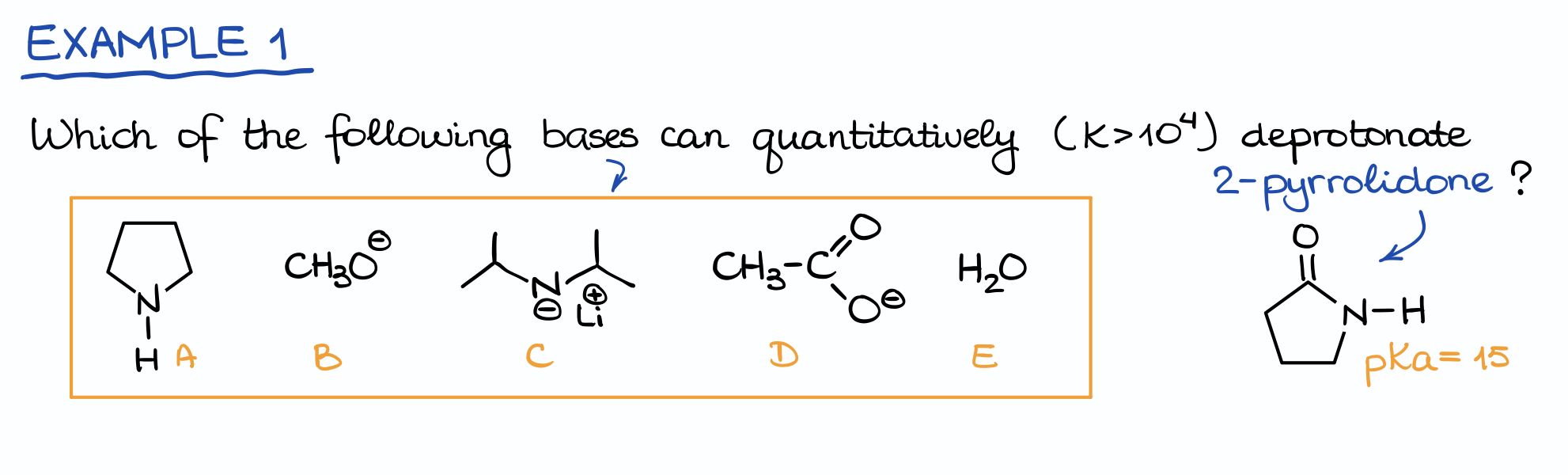
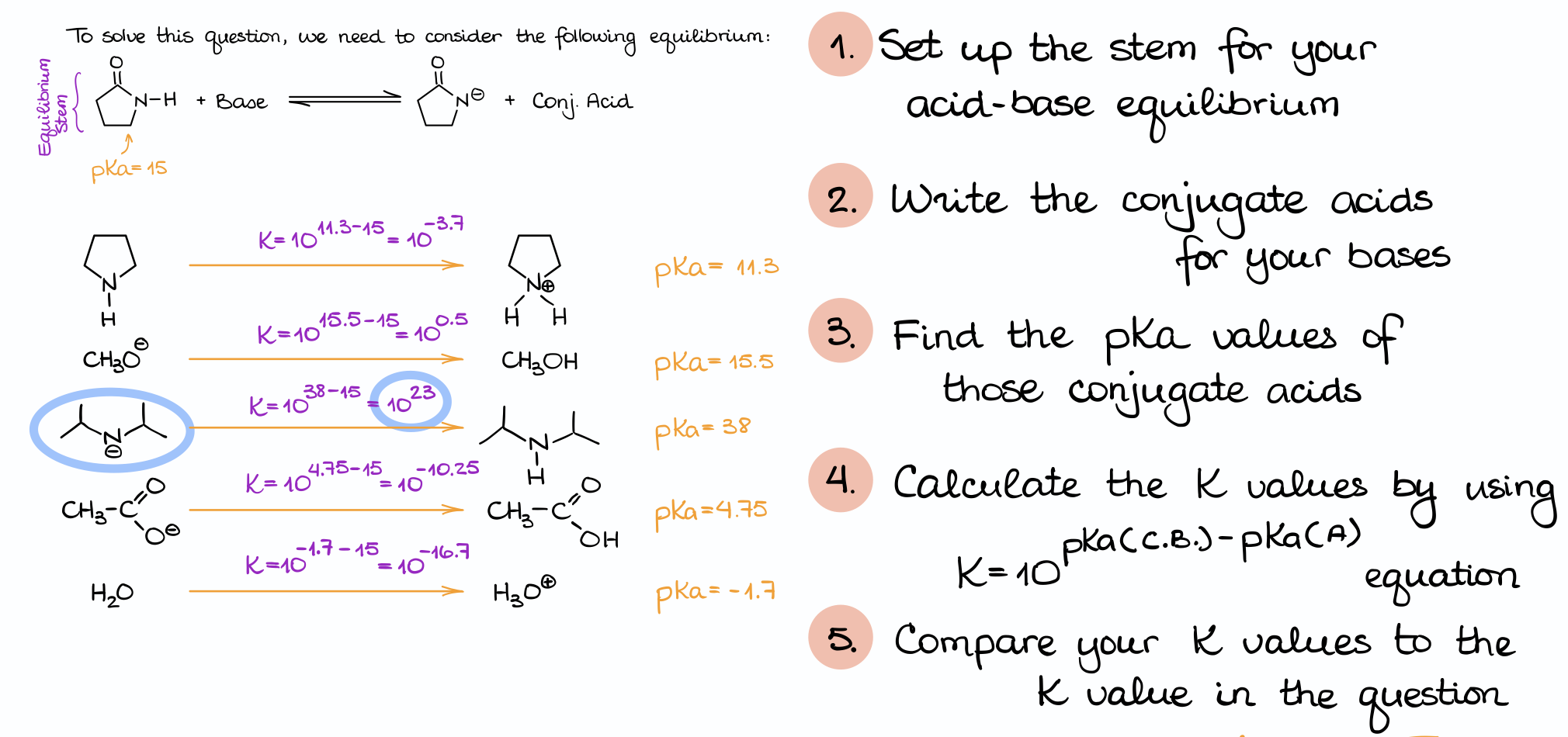
Here we have an acid (2-pyrrolidone) and a list of bases. And our task is to find which of these bases is strong enough to deprotonate our acid and give us the sufficient equilibrium constant.
Here’s how to approach a question like that:
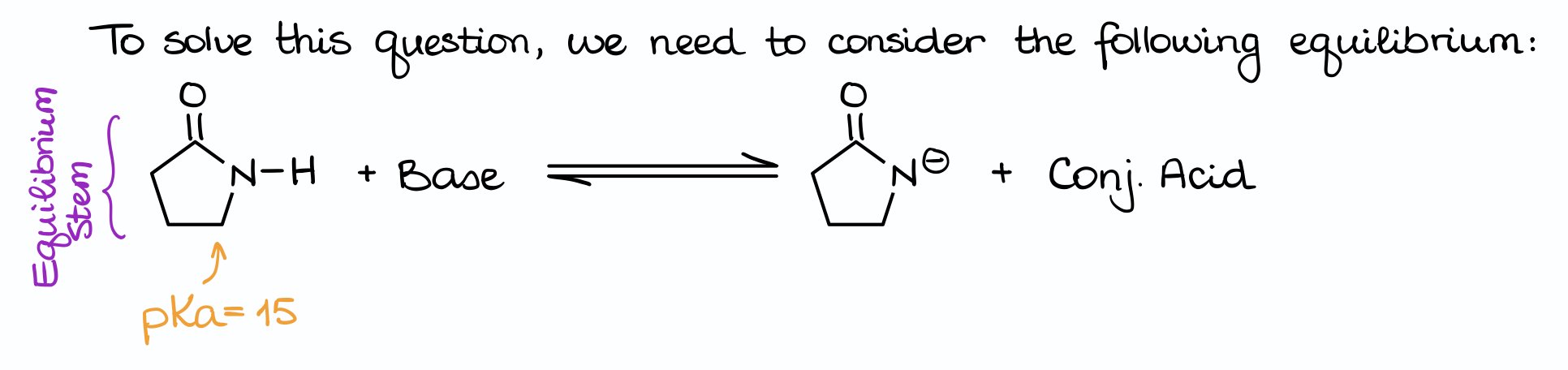
First, always make sure you set up your equation. It’s incredibly easy to mess up questions like that if you skip the equation part. Often, the reaction equation will not be there for you to see, so it’s up to you to draw it on the margins of your test or scratch paper. Make sure you read the question carefully too, so you understand what’s going on. In this case, we are deprotonating the pyrrolidone molecule, meaning that it acts as an acid by donating the proton. This way, we can say that our reaction looks like this, where we have our acid reacting with one of the bases giving us the conjugate base plus the conjugate acid.
Alright, so, we have the equation, now what?
Now, we are going to take each of those bases and convert them into corresponding acids by adding a proton to them.
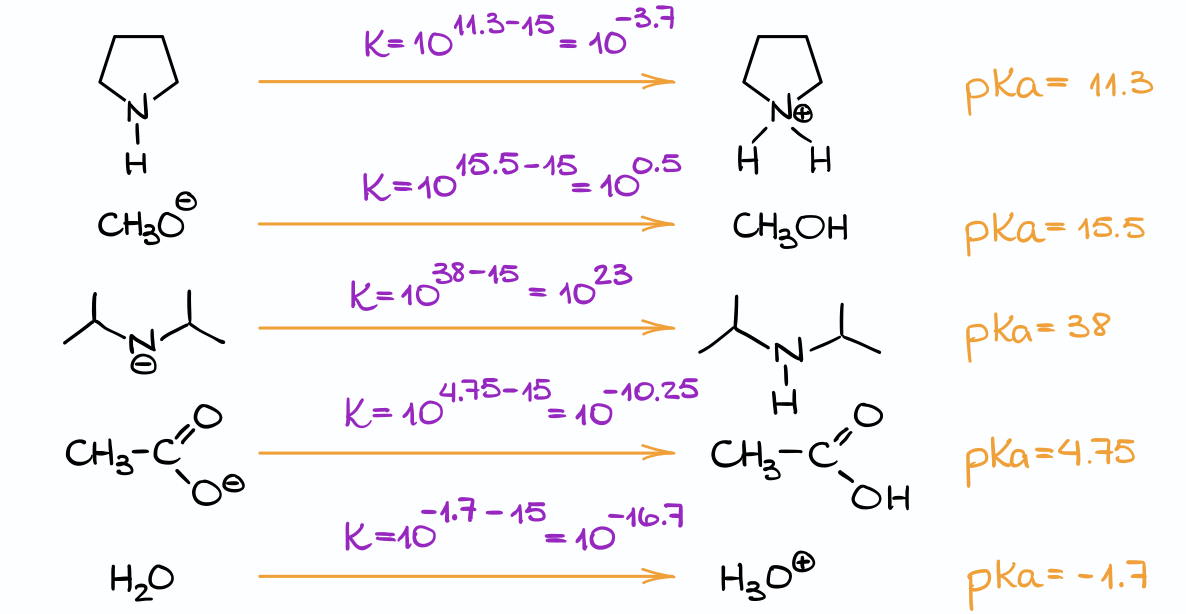
Once you have that, look up the pKa value for that species in the pKa table. You may not have the exact same species, so you’ll have to choose the closest species. Usually, that means you’ll need to choose something with the same functional group.
Once we have that, we can estimate the equilibrium constant using the technique we’ve used a moment ago. And, we’ll need to repeat this procedure for every base in our list.
Finally, once you have the equilibrium constant information for every base at hand, we can compare those numbers to what we have in the problem.
And in this case, only the base in option C suffices our conditions giving us the Keq greater than 104. Occasionally, you may have a situation when several species work, or you may have a few borderline cases. In such examples, you’ll probably need to choose the best one, aka the one with the larges or maybe smallest constant out of all of them. In any case, make sure you read the question carefully as the question itself will often contain useful hints on what you’re looking for.
To quickly recap the steps we’ve just used:
- We started with setting up the equation.
- We then wrote the conjugate acids for our bases.
- Then, we looked up the pKa values for those species.
- We then calculated the equilibrium constant for each reaction.
- And finally, we compared those to the equilibrium constant value in question to see which one fits the best.
I see a lot of students develop various shortcuts how to solve these types of questions. I encourage you to actually master the steps first before you develop your shortcuts. When you’re sitting in the room taking your exam, you may not have the shortcut in front of you, or you simply forget it. I cannot even count how many times students saw a question like this one on the practice test and sked me “so, are we looking for the pKa larger or smaller than the K?” And you know what my answer always is? I always say, “I have no idea, let’s do it step by step!” And this is simply because if you can’t remember the shortcut in the relaxed tutoring setting, you’re definitely not going to remember it in the more stressful exam scenario.
On top of that, what if I change the wording or the premise of the question a little. What shortcut are you going to use then? I hope you get the point.
Example 2
Here’s an example asking a very similar type of a question. Soooo, are we looking for the pKa higher or lower than 106? I have no idea, let’s do it step by step!
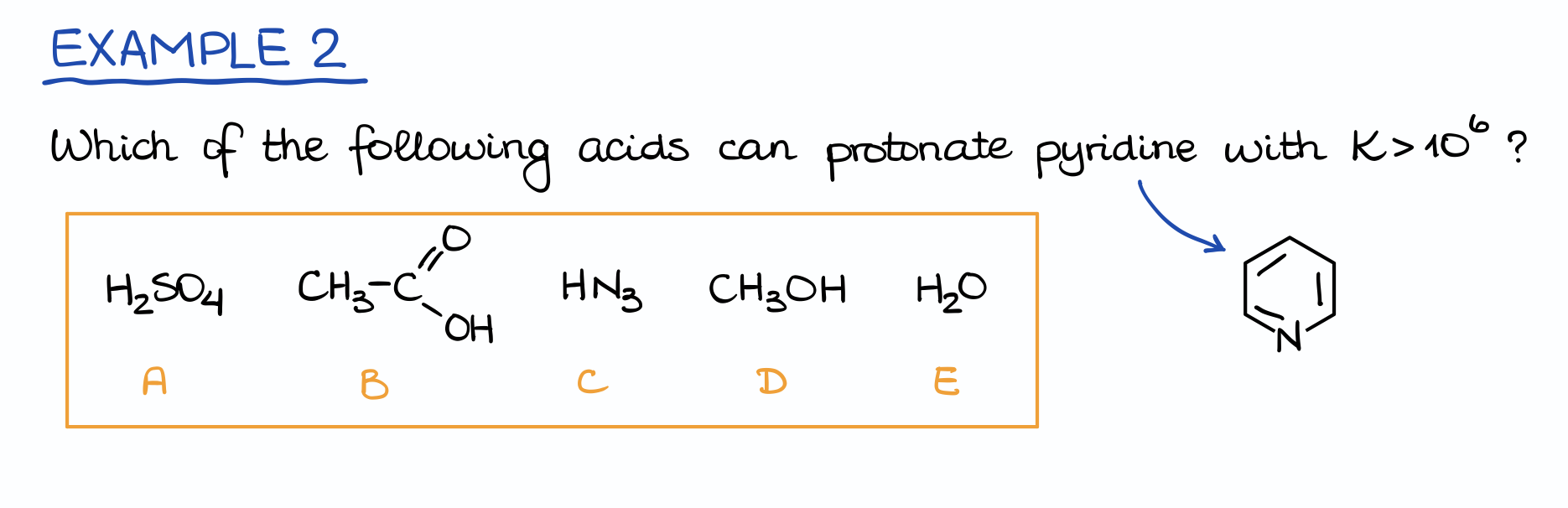
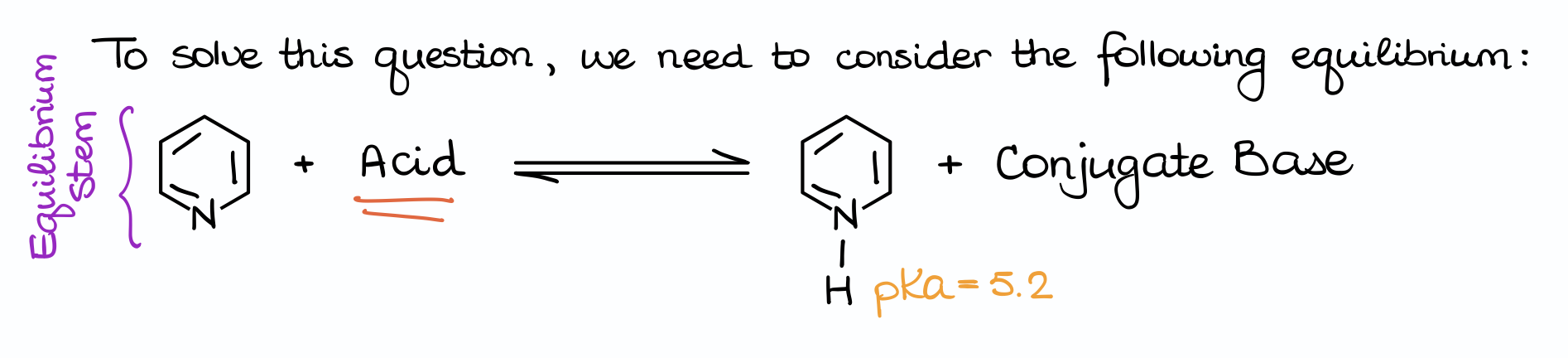
First, we’re going to set up the equation. Notice how in this case our molecule (pyridine) is acting as a base by being protonated. So, we’ll have the same conjugate acid in each case (the protonated pyridine), but the actual acid will change every single time.
Next, I’m going to write out all of my acids, and look those up in the pKa table.
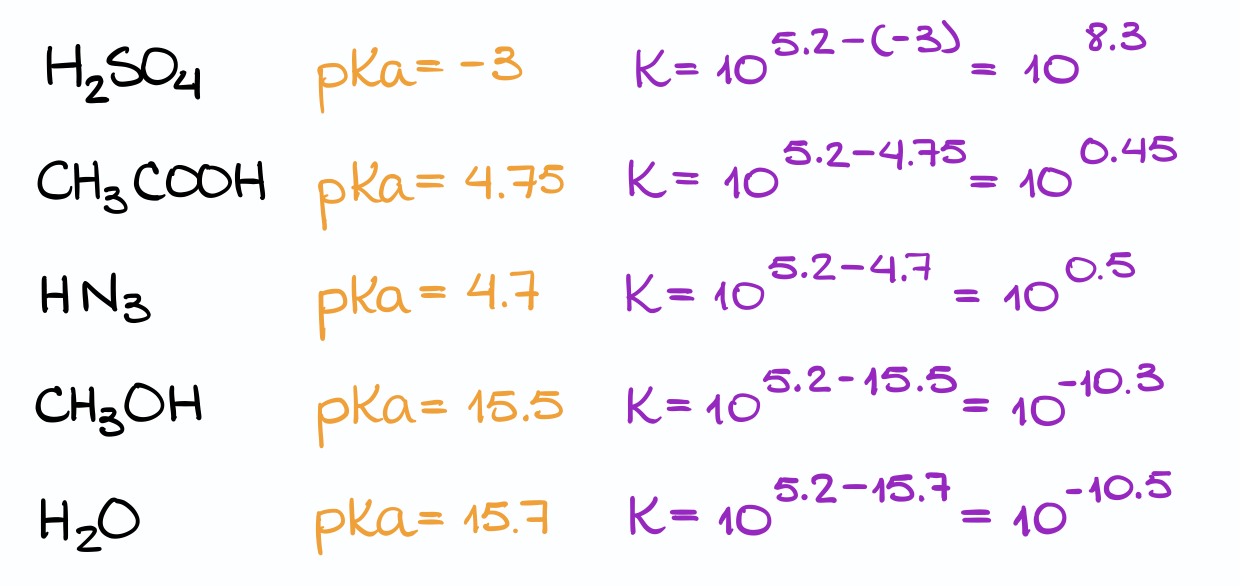
I’ll then calculate the equilibrium constant for each of those reactions. And finally, compare it to the value in question to find which acid works the best.
While these questions may seem a little tedious, they are not that bad. Once you solve a few of those, you’ll get a hang of them and start cracking them easily. Practice is the key here, so don’t just think you’ll be able to do this on the test, practice now. And do enough practice to make sure you don’t even think about the steps. You want to be working through these steps automatically.
Ranking Acids and Bases According to Their Strength
Alright. Now, how about the ranking questions?
Ranking Acids
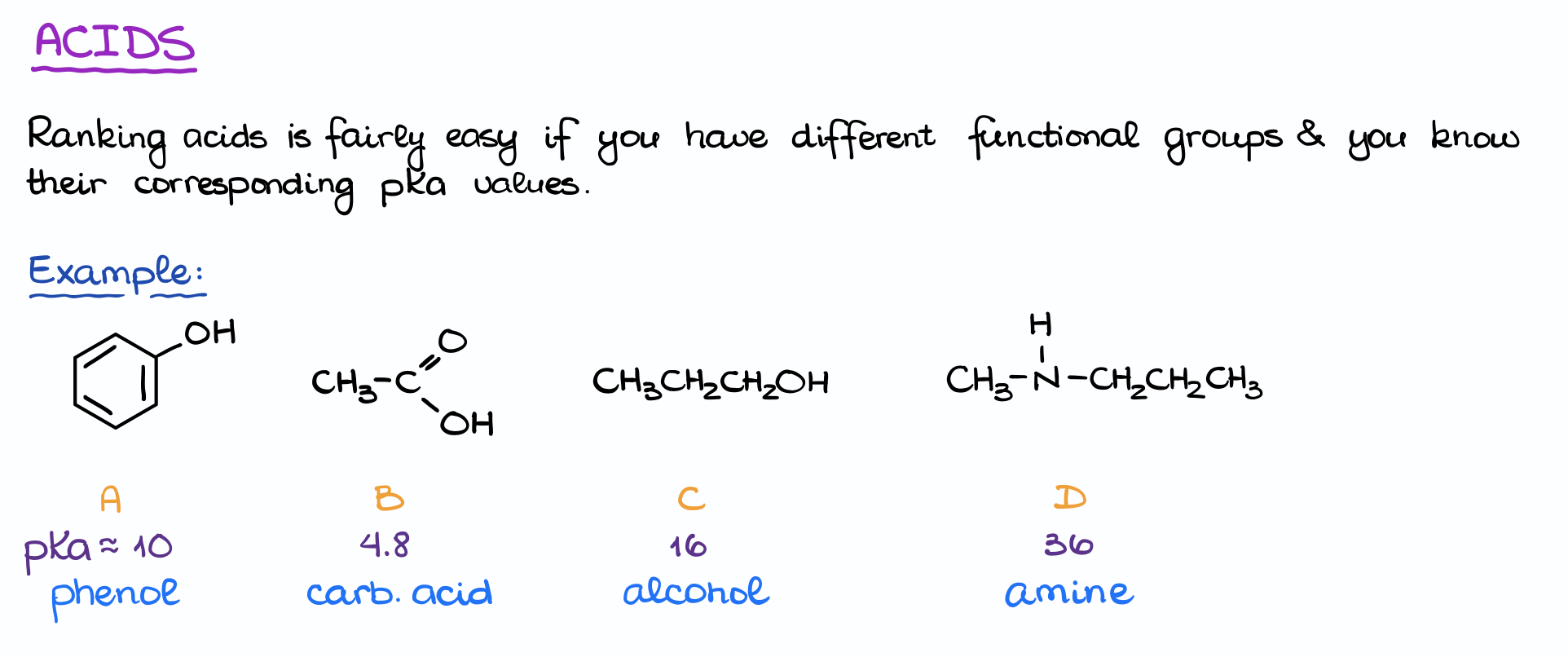
Ranking acids according to their strength is actually quite straightforward. You just need to know the pKa values and you’re good to go.
Here I have an example where we want to rank the following species according to their acidity from the weakest to strongest.
The only thing I need to know to do is the pKa values from the pKa table. So, I look those numbers up and compare them. Since we know that the higher the pKa value, the weaker the acid is, we can easily rank these species in the following manner:
The carboxylic acid, having the pKa of 4.8 is the strongest of them all. So, it’s going to be at the very end of my list. And the amine with the pKa of 36 is the weakest of them in terms of acidity. So, it’s going to be at the very beginning of this list as the weakest acid.
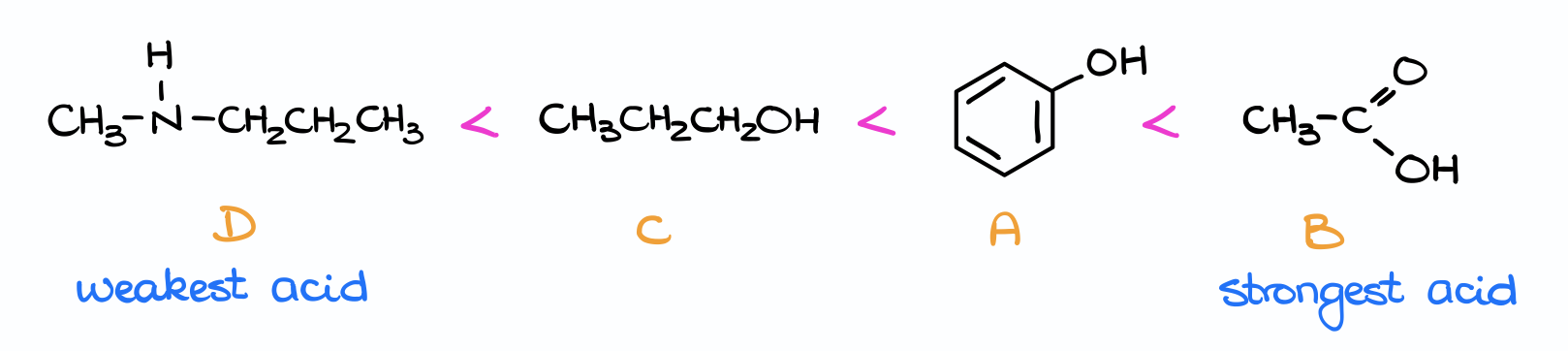
Ranking Bases
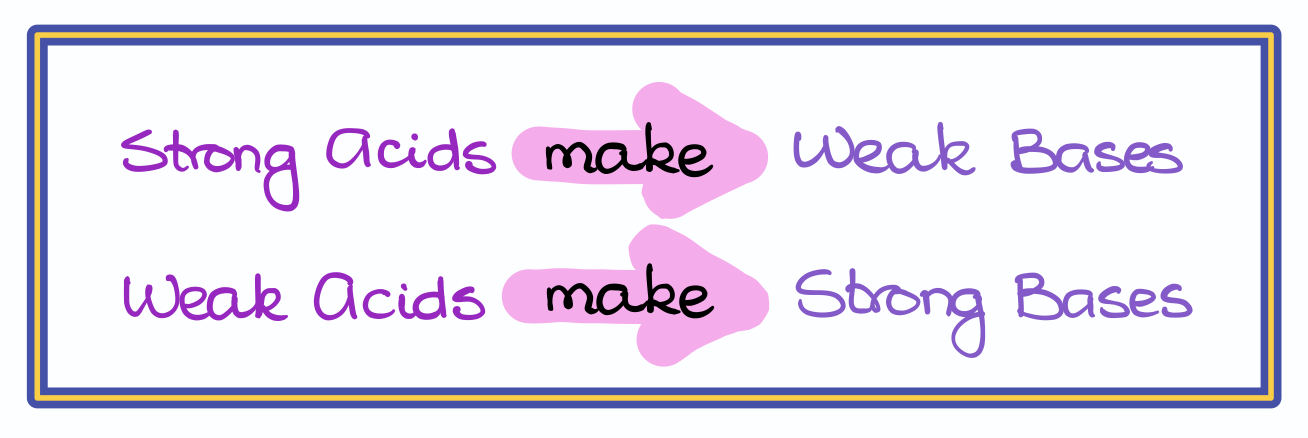
When it comes to bases though, it’s a little more complicated than that. As we cannot compare the pKa values for the bases, we are going to compare the pKa value for the corresponding conjugate acids. And here it’s important to remember that strong acids come from weak bases while weak acids come from strong bases.
Let me illustrate this with an example:
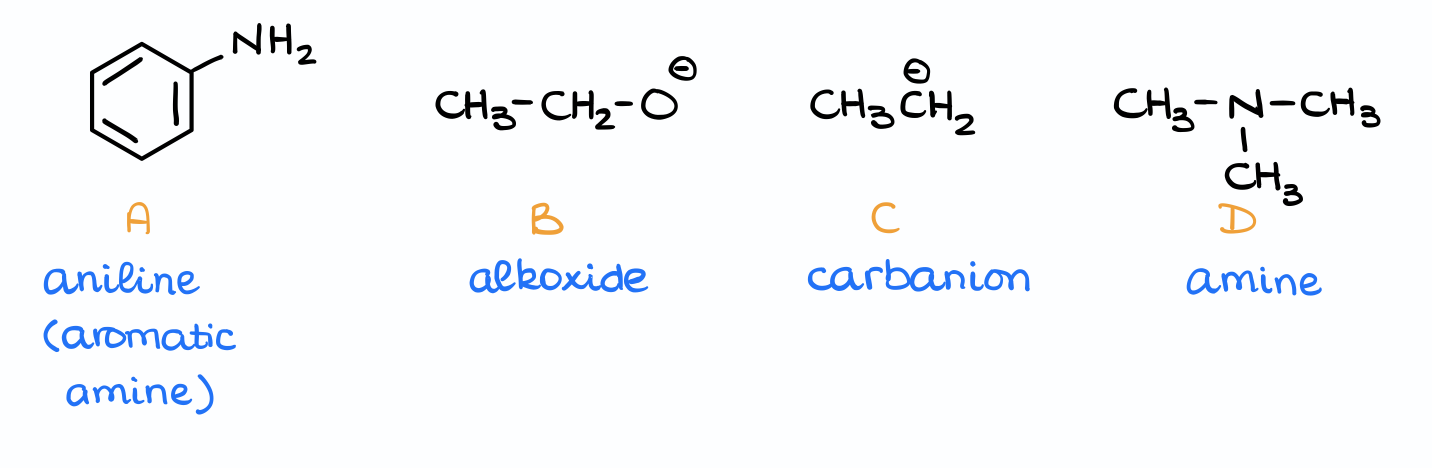
Here we have four different bases. The first thing I’m going to do is to add a proton to each of them to make the corresponding conjugate acids. Now, I can look up the pKa values for those molecules.
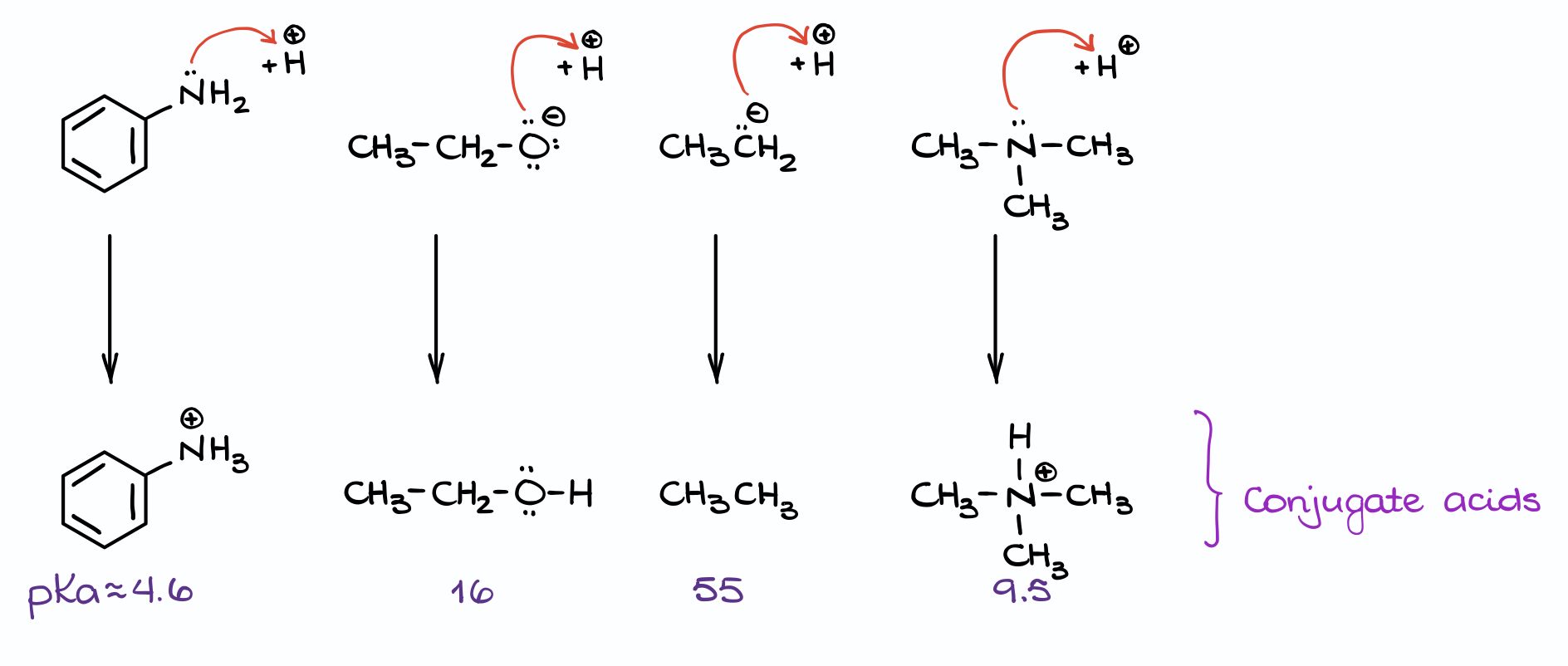
Finally, by comparing the pKa values we can identify the stronger and weaker acids. And, by extension, this gives us the stronger and weaker bases.
For instance, the first example here has the lowest pKa value out of all those acids. So, it’s the strongest acid, which means it was made by the weakest base. Likewise, an alkane in the third example is the weakest acid, which means it came from the strongest base.
Thus, we can rank these molecules from the weakest base to the strongest base like this:
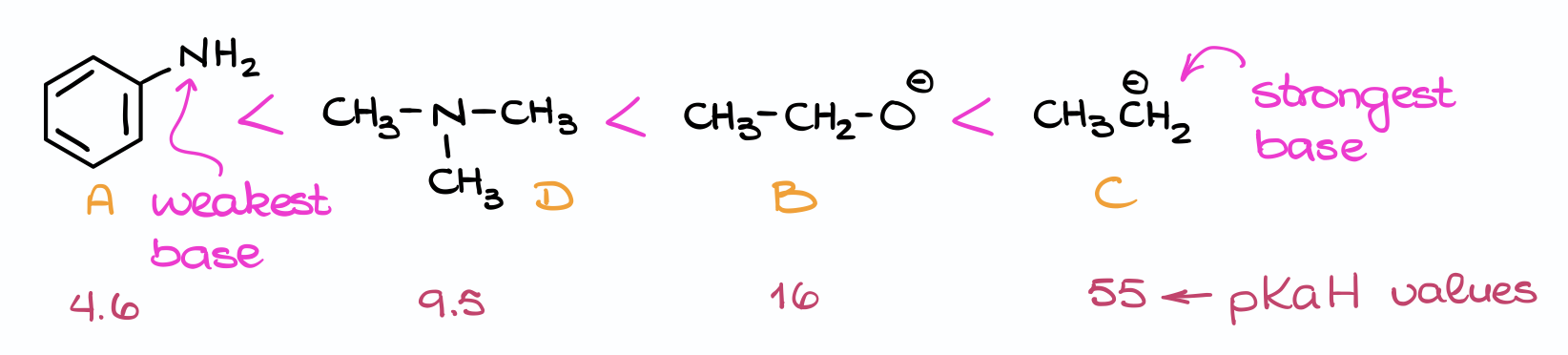
So, as you can see, you can use the pKa table to answer a whole range of questions regarding the acid-base equilibrium. In the next lesson in this series, we’ll talk about how to approach similar questions without the pKa table by just looking at the molecules. So, make sure you check it out to become a true master of the acid-base equilibrium!
