Acetals Formation and Hydrolysis
Acetals are incredibly common in nature and are important for organic chemistry synthesis. The acetal formation is also one of those mechanisms that instructors love to test students on. So, let’s put this reaction under our imaginary microscope and go over the ins and outs of the mechanism, conditions, and the easy trick that will save you a ton of time on the test.
What are the Acetals?
First of all, what exactly are acetals? Chemically speaking, an acetal is a functional group where you have two -OR groups sitting on the same carbon. So, it might look like a di-ether of some sort, but it is not! We know that a distinctive feature of a functional group is its unique chemical properties. Well, ethers and acetals couldn’t be further apart from each other in terms of their chemistry! So, you should never count an acetal as an ether.
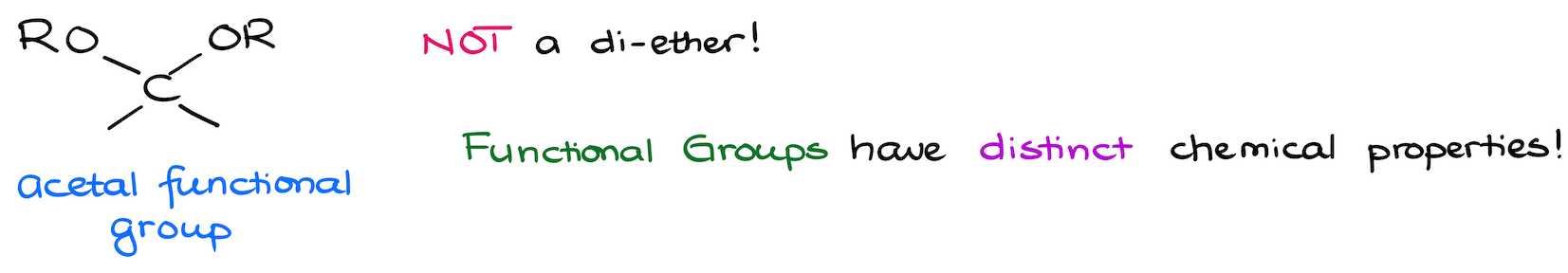
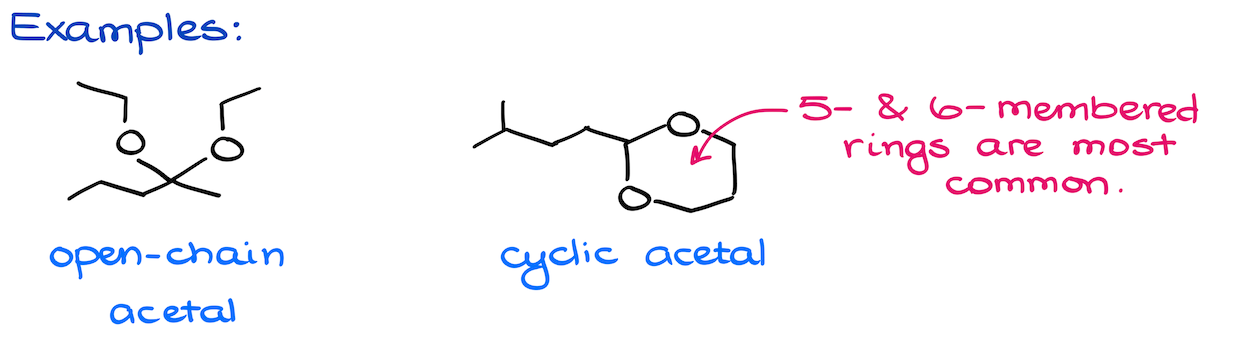
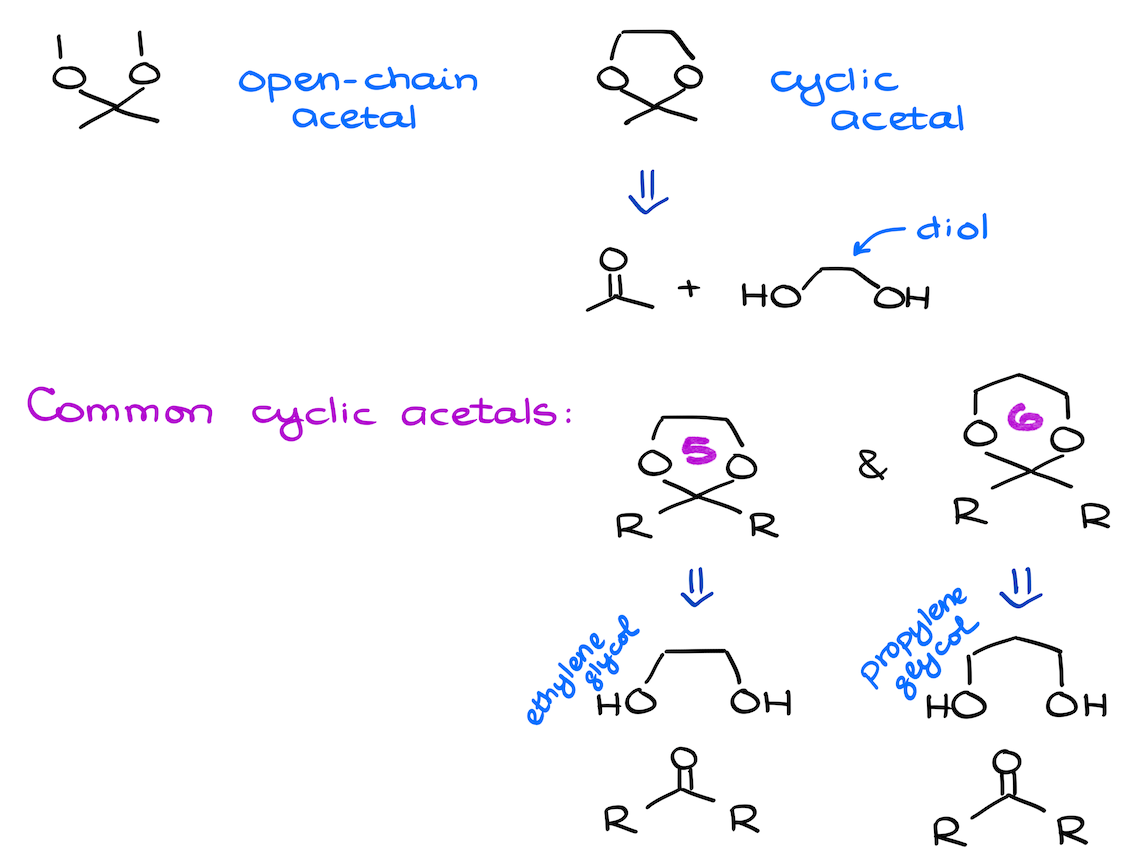
Acetals can be open-chain or cyclic. Chemically speaking, there’s no difference between those. Cyclic acetals form a little easier if they make 5- or 6-membered rings. But other than that, there’s no other difference that I can point out between those.
There’s also one other thing you need to know about the acetals. And that is they sometimes they are called “ketals” by some older books and instructors. According to the current IUPAC rules and recommendations, an acetal is the umbrella term, and a ketal is a subtype of an acetal. If your instructor insists on calling ketone derivatives “ketals” and aldehydes derivatives “acetals,” well, go with it, I suppose.
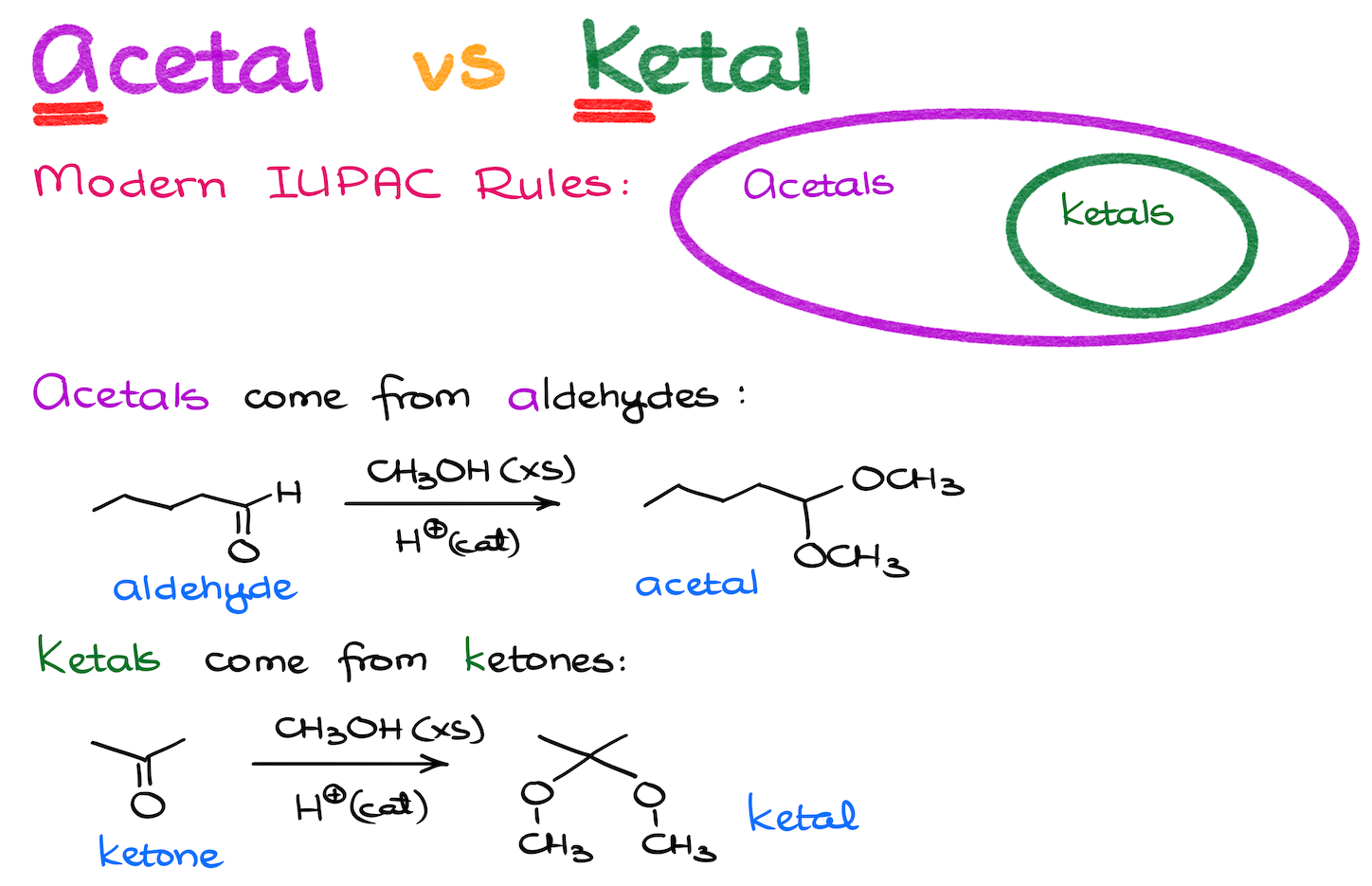
So, it’s not the name that matters. But some instructors are very particular about it for whatever reason.
Acetals vs Hemiacetals
So now, we know what acetals and ketals are. But you’re also going to hear another term, which is “hemiacetal.” As the name suggests, it’s a “half-way” acetal. Hemiacetals have one -OR group and one -OH attached to the same carbon. So, they look like they are a combination of an alcohol and an ether. But just like in the case of the acetals, hemiacetals are their own functional group.
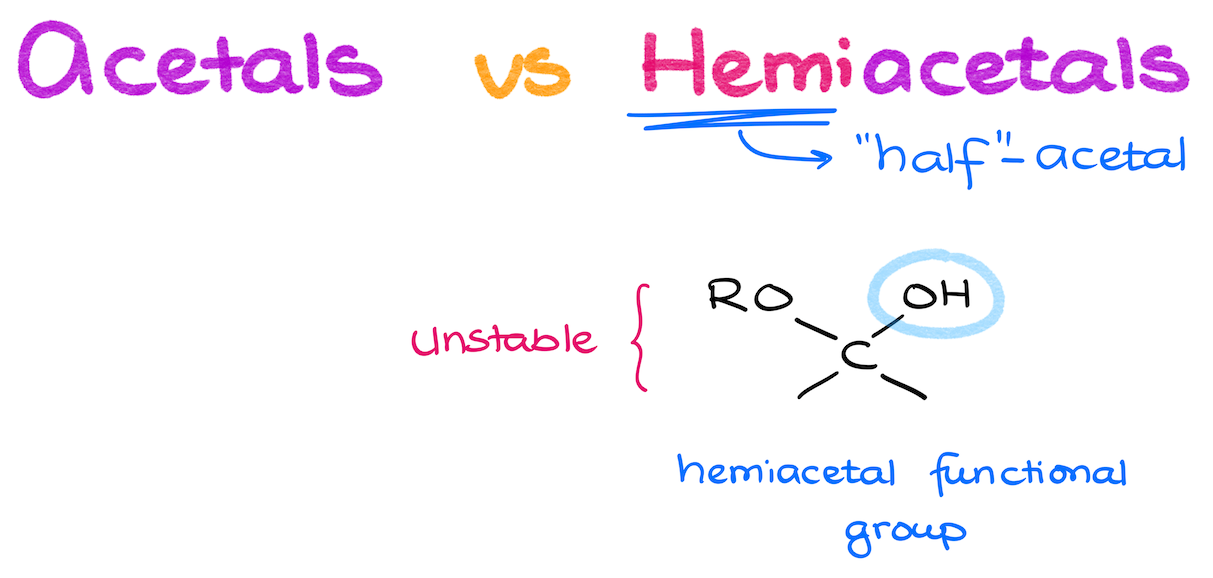
We’re not going to pay too much attention to the hemiasetals though, as those species are typically unstable and only show up at equilibrium with the corresponding carbonyls, or as an intermediate in the acetal formation mechanism. They will also show up more in the future chapters in your course, once you start talking about carbohydrates (if you’re covering those in your course to begin with).
How do me make Acetals?
We make acetals by reacting a corresponding carbonyl (an aldehyde or a ketone) with an excess of an alcohol in acidic media. Acid is a catalyst in this reaction, and it is very important for it. Acetal formation is not possible in neutral or basic conditions.
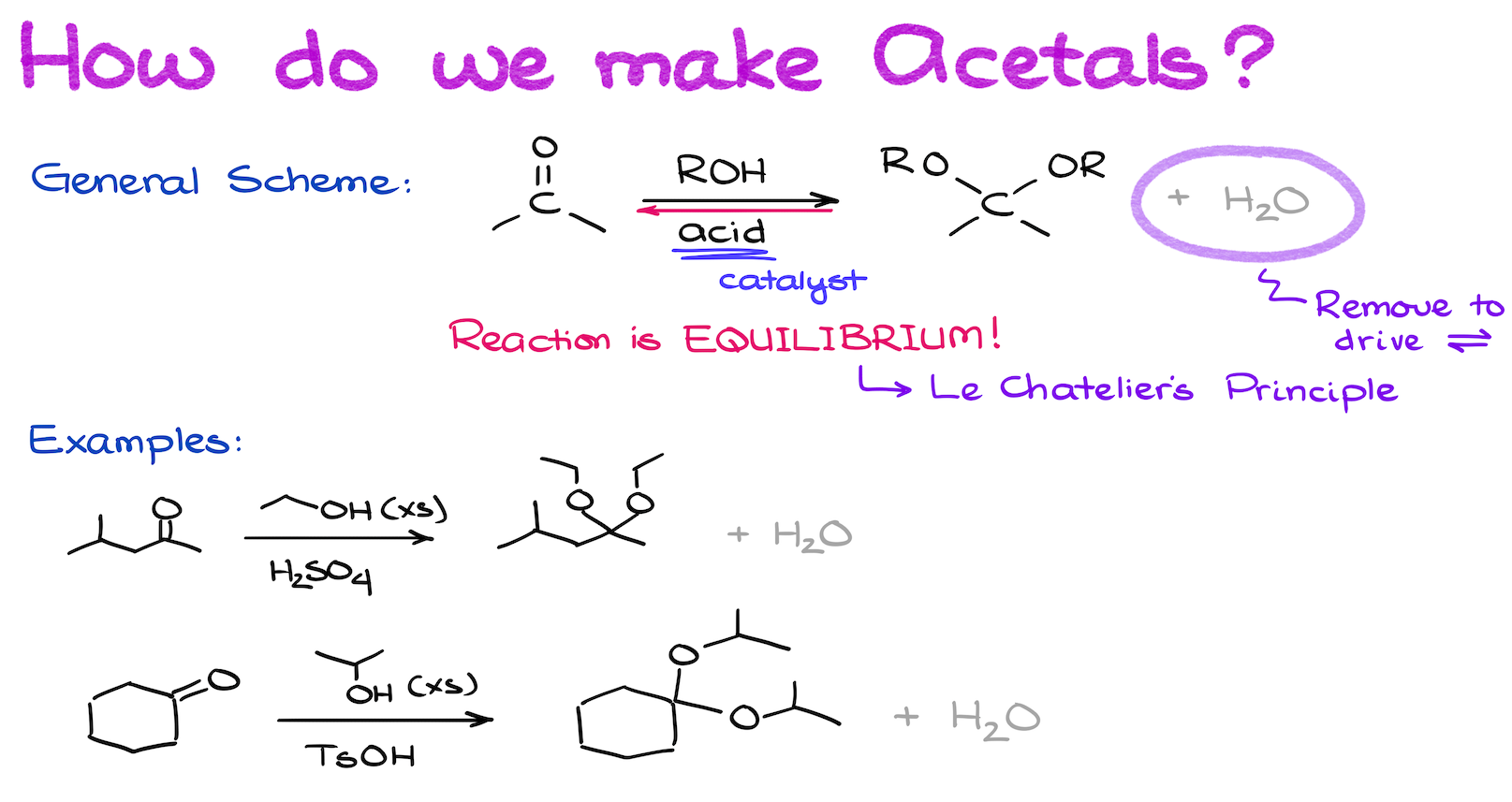
Moreover, acetal formation is an equilibrium. We drive this equilibrium using the Le Chatelier’s principle by either adding excess of the starting material (typically alcohol), or — more commonly — removal of water. We can continuously remove water by either using the azeotropic reflux — effective, yet somewhat outdated technique, or by using the 4Å molecule sieves, which physically trap water and drive your reaction to completion.
For the exam purposes, it’s irrelevant what you choose to write. Most of the time, we don’t even talk about how we actually perform this reaction in the course. So, you most likely won’t even have to mention it unless your instructor is very particular about the realistic reaction conditions when you’re writing your equations.
Mechanism of the Acetal formation
Now, the fun part is the mechanism of the acetal formation. The entire mechanism is an endless parade of proton transfer steps, nucleophilic attacks, and leaving group dissociations.
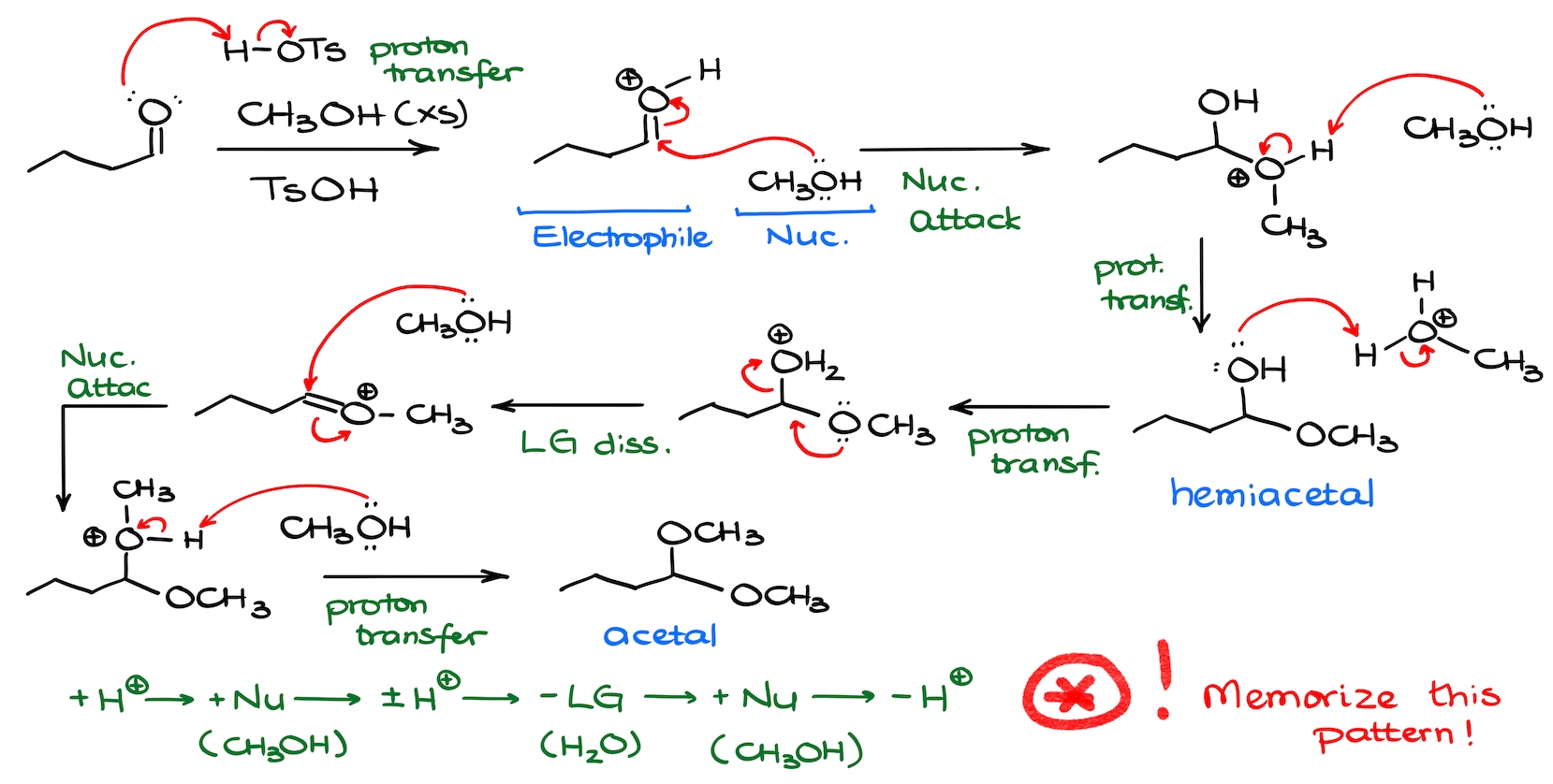
The best way to practice the mechanisms like this one, is to find the underlying pattern that your steps follow in the mechanism.
Now, as I’ve mentioned above, acetals can be open chain like in the previous example, or cyclic. If you’re reacting your carbonyl with a diol, then you’re likely going to make a cyclic acetal. Keep in mind though — this reaction like any other cyclization favors the formation of the 5- and 6-membered rings. So, most common diols you’re going to see in this type of acetal formation are the ethylene- and propylene glycols and their derivatives. The former makes a 5-membered ring, the latter makes a 6-membered ring.
In terms of the mechanism, we have the same pattern here as in the previous example.
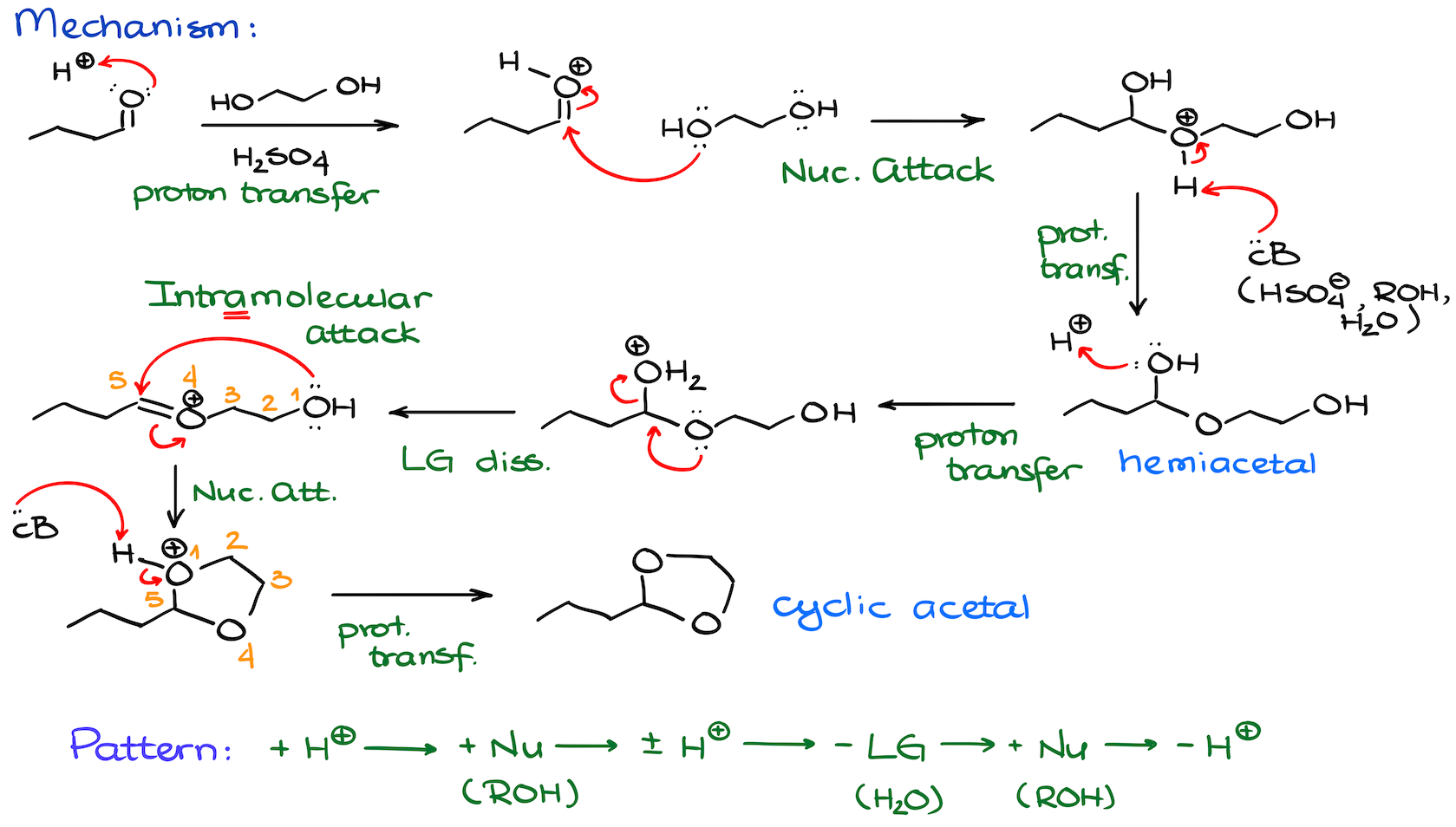
The only difference is that instead of the nucleophilic attack by the second equivalent of our alcohol, we’re going to do the intramolecular nucleophilic attack closing up the ring.
This is a long mechanism, I understand. But trust me, you wanna practice it to the point where you can write it easily for any kind of compound in front of you. This general pattern of proton transfers and nucleophilic attacks is also going to show up frequently throughout the entire chemistry of carbonyl compounds including carboxylic acids and their derivatives. So, a few extra minutes of practice now will pay off later. On top of that, this mechanism is a fairly common exam question. So, chances are, you’re going to have to write it out during your next test in all details!
Trick!
Now, at the beginning of this tutorial I promised you a trick. The thing is, being able to draw the mechanism is very important. But being able to quickly visualize the product without going through every single step of the mechanism is equally important. So, here’s how we’re going to be able to quickly visualize the product for this reaction:
- Redraw your carbonyl and put your alcohols right next to it from the carbonyl’s oxygen side.
- Erase the carbonyl and the hydrogens of the -OH groups of the alcohols.
- Connect the oxygens of the alcohols to the carbon that used to have the carbonyl.
And that’s it! We have our product. This trick works for both open-chain and cyclic acetals. So, the next time you’re working on the acetal formation reaction, use the trick first to quickly predict the product, and then confirm the structure of your product by drawing the mechanism. With more practice, you’ll be able to do it in your head without actually having to redraw the starting materials and then erasing parts of those molecules.
Acetal Hydrolysis
As I’ve mentioned above, acetal formation is a reversible equilibrium. We make acetals by reacting carbonyls with alcohols. We drive this equilibrium towards the product by removing water. However, since it’s an equilibrium, by adding excess water to the acetal, we’ll drive the equilibrium backwards breaking up (aka, hydrolyzing) our acetal and making the corresponding carbonyl and alcohol back.

And since this reaction is an equilibrium, we’re going to have all the same intermediates in the acetal hydrolysis as in the acetal formation.
Mechanism of the Acetal Hydrolysis
I’ll remind you here, that this reaction requires acidic catalysis. So, we’ll have some sort of an acid that will serve as a source of a proton in our system. Typically, we’re going to use either sulfuric or tosylic acids. But those are, of course, not the only options you might see in your course or on the test. So, keep your eyer peeled so you don’t confuse the acid catalyst with a different reagent.
Mechanistically speaking, the acetal hydrolysis is a combination of all the same proton transfer, nucleophilic attack, and leaving group dissociation steps as in the acetal formation, but in reverse.
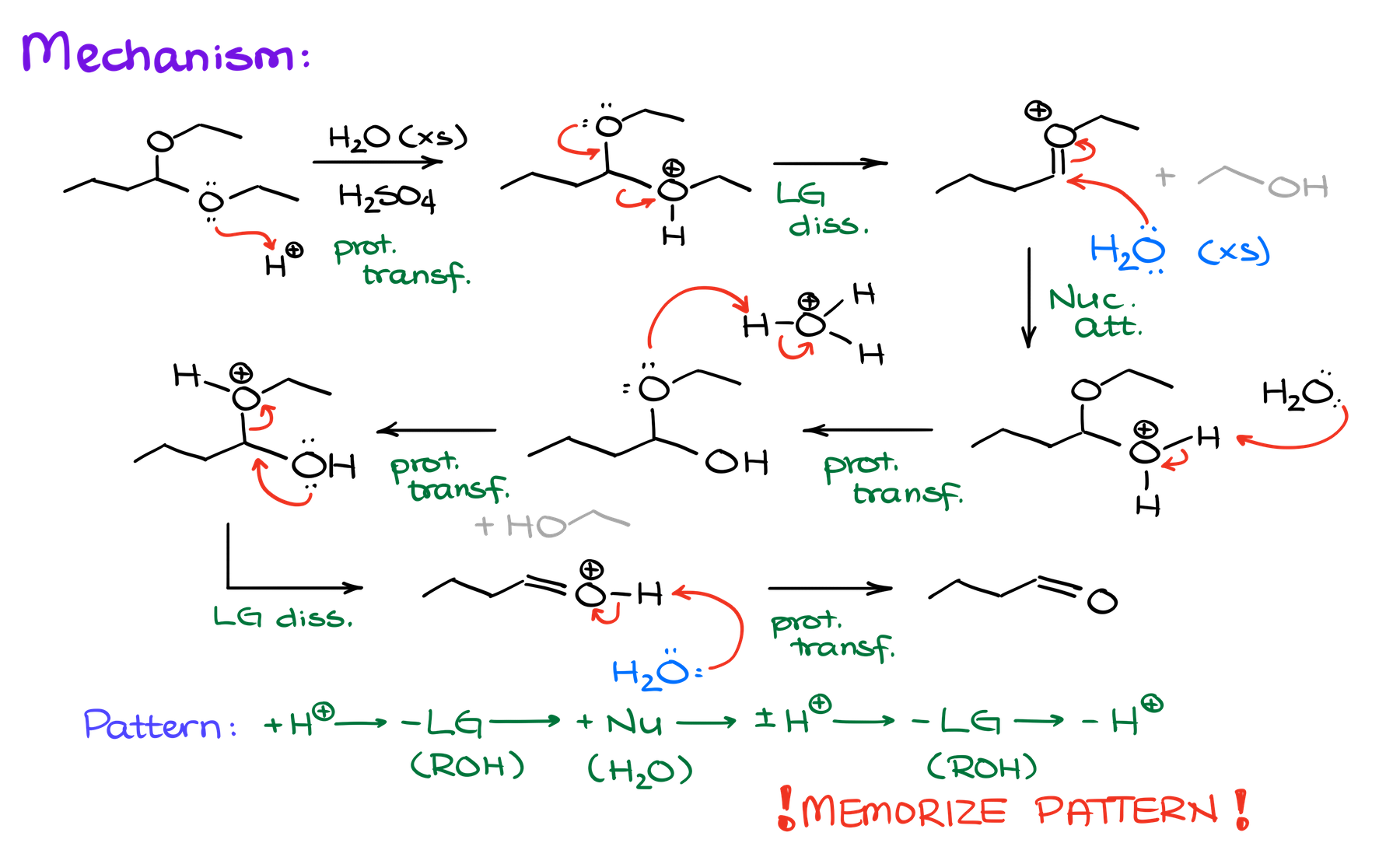
And here’s another example of a cyclic acetal hydrolysis:
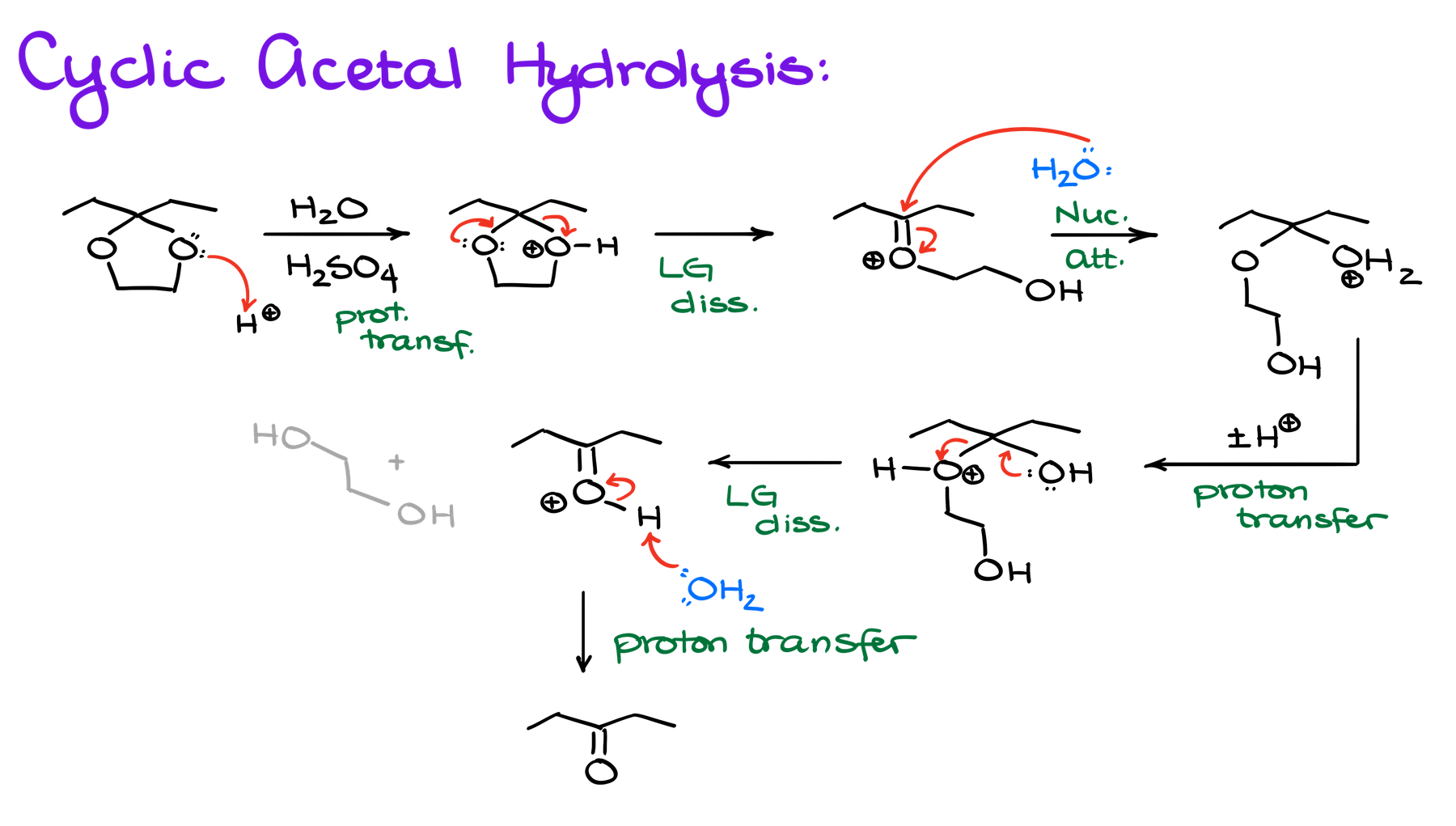
Just like in my last video on the acetal hydrolysis, I’m only showing one directional arrow in each of my steps instead of the equilibrium arrow to specify the direction in which I wanna take my reaction. But as I’ve mentioned before, if your instructor is particular about the equilibrium arrows, make sure you’re using those instead of a single direction arrow like what I’m doing here.
Trick!
Conveniently enough for us, just like in the case of the acetal formation, theres a simple trick that we can use to easily predict the products of the acetal hydrolysis.
- First, redraw your acetal as is.
- Then, erase the bonds between the carbon and the oxygens of the acetal. Keep the oxygens though, we’ll need them.
- Add a C=O bond to the carbon that used to be attached to your oxygens.
- And finally, add hydrogens to each of the oxygens that you have hanging there, and you’re done!
As you can see, predicting the products in the acetal hydrolysis is just as easy as in the acetal formation. So, do plenty of practice to make sure you can do it quickly and correctly on the test!
