27. Synthesis of a Complex Ketone
I have a pretty cool synthesis challenge here for you.
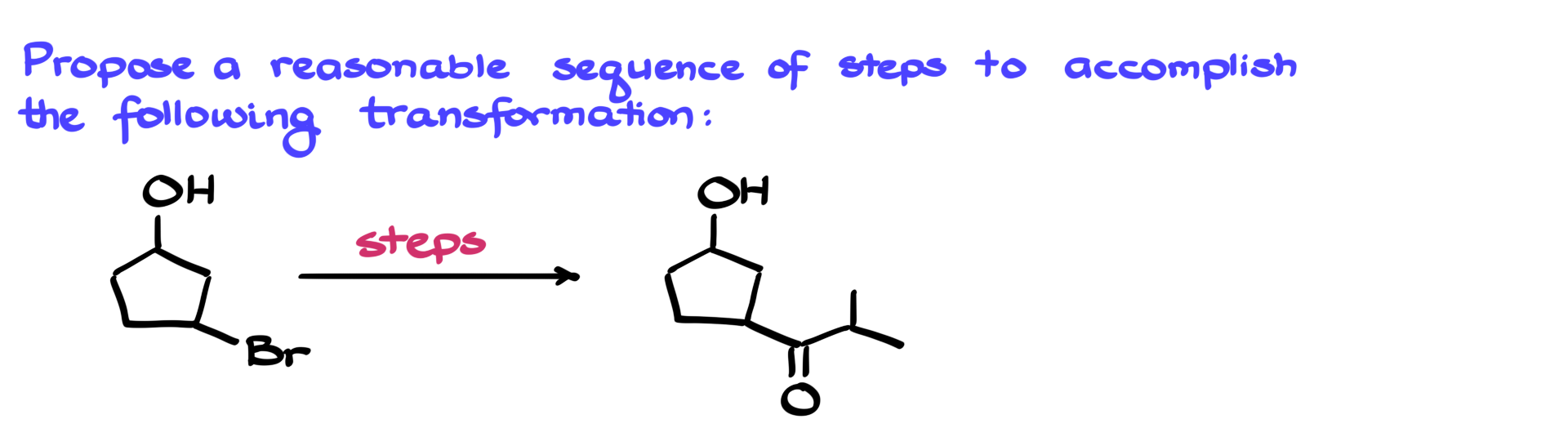
As always, I’m going to start by analyzing my starting material and my product to figure out what changes have occurred and to get some ideas about how to approach this synthesis.
First, I notice that my starting material contains two distinct functional groups: an alkyl halide and an alcohol. Looking at my target molecule, I see that a new carbon-carbon bond has been formed. Another important observation is that the alcohol remains unchanged throughout the synthesis. This strongly suggests that the alcohol had to be protected during the reaction sequence.
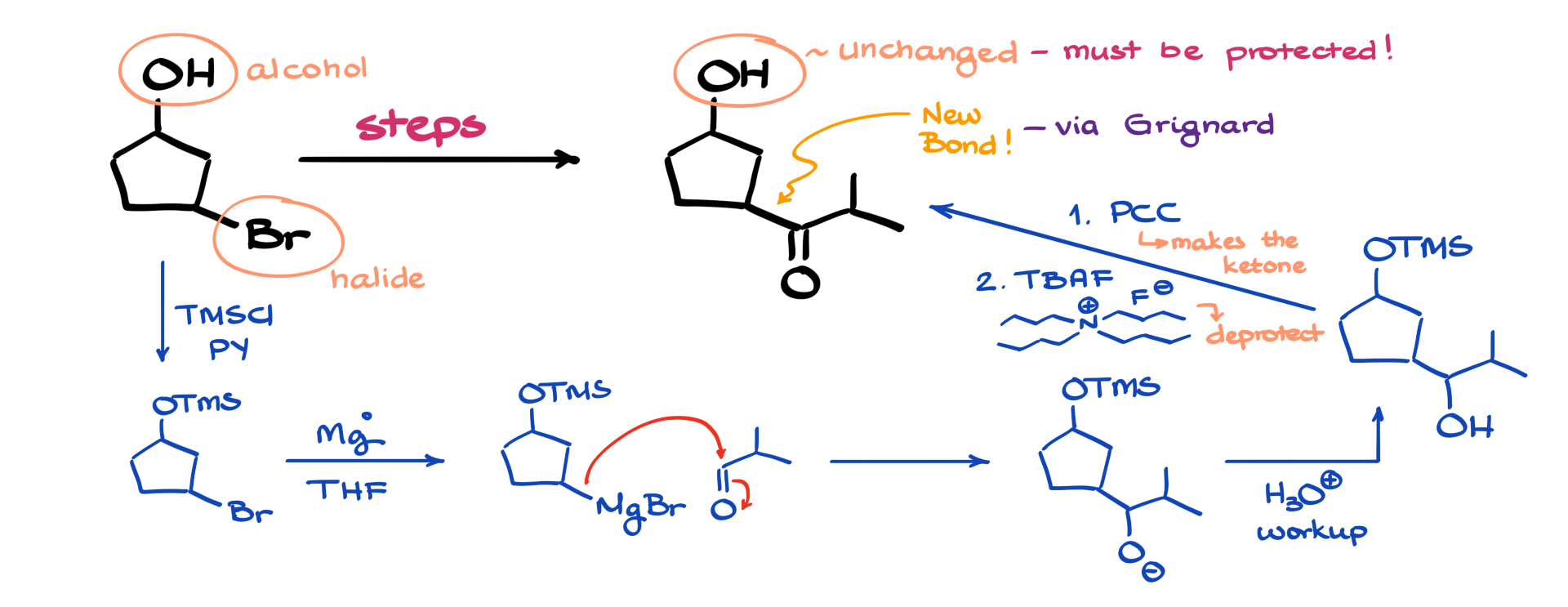
One key consideration is that carbon-carbon bonds are often made using Grignard reactions, but Grignard reagents are incompatible with alcohols or any other acidic functional groups. This further supports the idea that protecting the alcohol is a necessary first step.
To begin, I will protect the alcohol using a trimethylsilyl (TMS) protecting group. TMS protection is a great choice here—it’s versatile, easy to add and remove, and stable under most reaction conditions. With the alcohol safely protected, I can now focus on converting the alkyl halide into a Grignard reagent.
Next, I generate my Grignard reagent from the alkyl halide by treating it with magnesium metal in dry ether. To use this Grignard reagent for carbon-carbon bond formation, I will introduce an aldehyde as my electrophile. The Grignard reagent will attack the carbonyl of the aldehyde, leading to the formation of an alkoxide intermediate. After an acidic workup, this alkoxide is protonated, yielding a secondary alcohol.
However, my final product is not an alcohol—it contains a carbonyl group, meaning I need to oxidize the secondary alcohol. This oxidation step can be performed using PCC (pyridinium chlorochromate) or any other mild oxidant. Since secondary alcohols can only be oxidized to ketones, the choice of oxidizing reagent doesn’t really matter, but PCC is a concise option to write out, so I’ll go with that.
Once the oxidation converts my alcohol into the desired ketone, the final step is to remove the protecting group. This can be accomplished using tetrabutylammonium fluoride (TBAF) or any other fluoride source, which selectively cleaves the TMS group, regenerating the free alcohol.
So, the key steps in this synthesis were recognizing that the alcohol needed to be protected, identifying the type of Grignard reagent to use, and strategically forming the main carbon-carbon bond. Other than that, I think this was a pretty straightforward synthesis—but let me know in the comments if you have alternative approaches! I’d love to hear your ideas.
