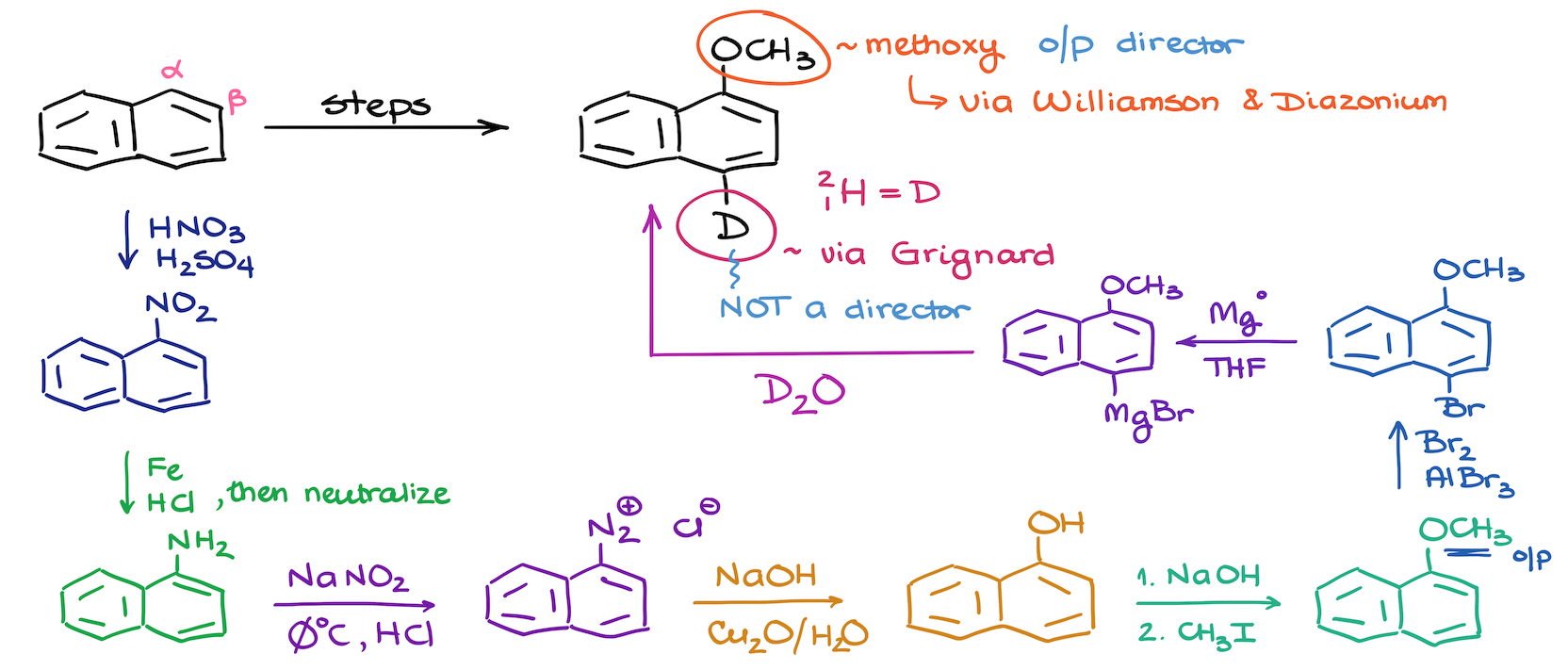
19. Synthesis of a Deuterated Naphthalene
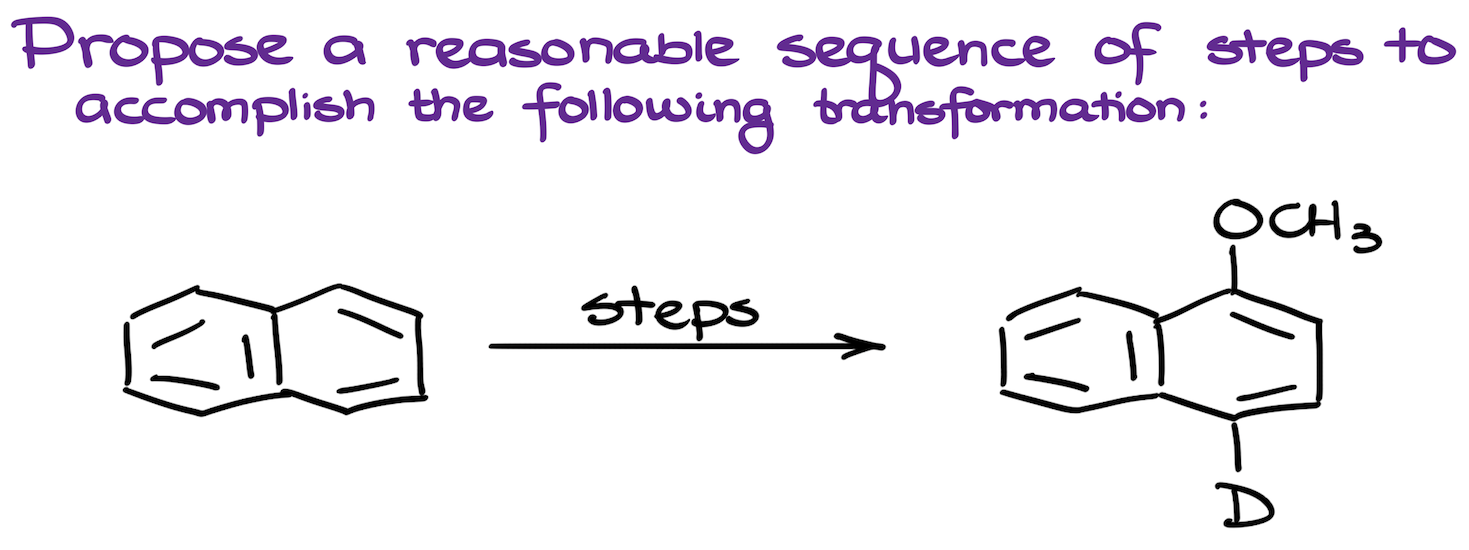
The synthesis involves modifying a naphthalene molecule by adding a methoxy group and a deuterium atom. While we don’t typically talk much about the deuterium labeling in a sophomore organic chemistry course, some instructors like it. So, it’s a good challenge.
The methoxy group is introduced using a Williamson ether synthesis strategy, while the deuterium is incorporated through a Grignard quench method. The synthesis begins with the nitration of naphthalene, followed by the reduction of the nitro group to an amine, which is then converted into a diazonium salt. This salt undergoes a substitution to form a phenol, which is then transformed into an ether.
For the deuterium addition to the molecule, we first do halogenation to introduce a halide, which is then reacted with a Grignard reagent and deuterated water to incorporate deuterium. The process involves multiple steps and careful consideration of the directing effects of substituents on the aromatic ring.