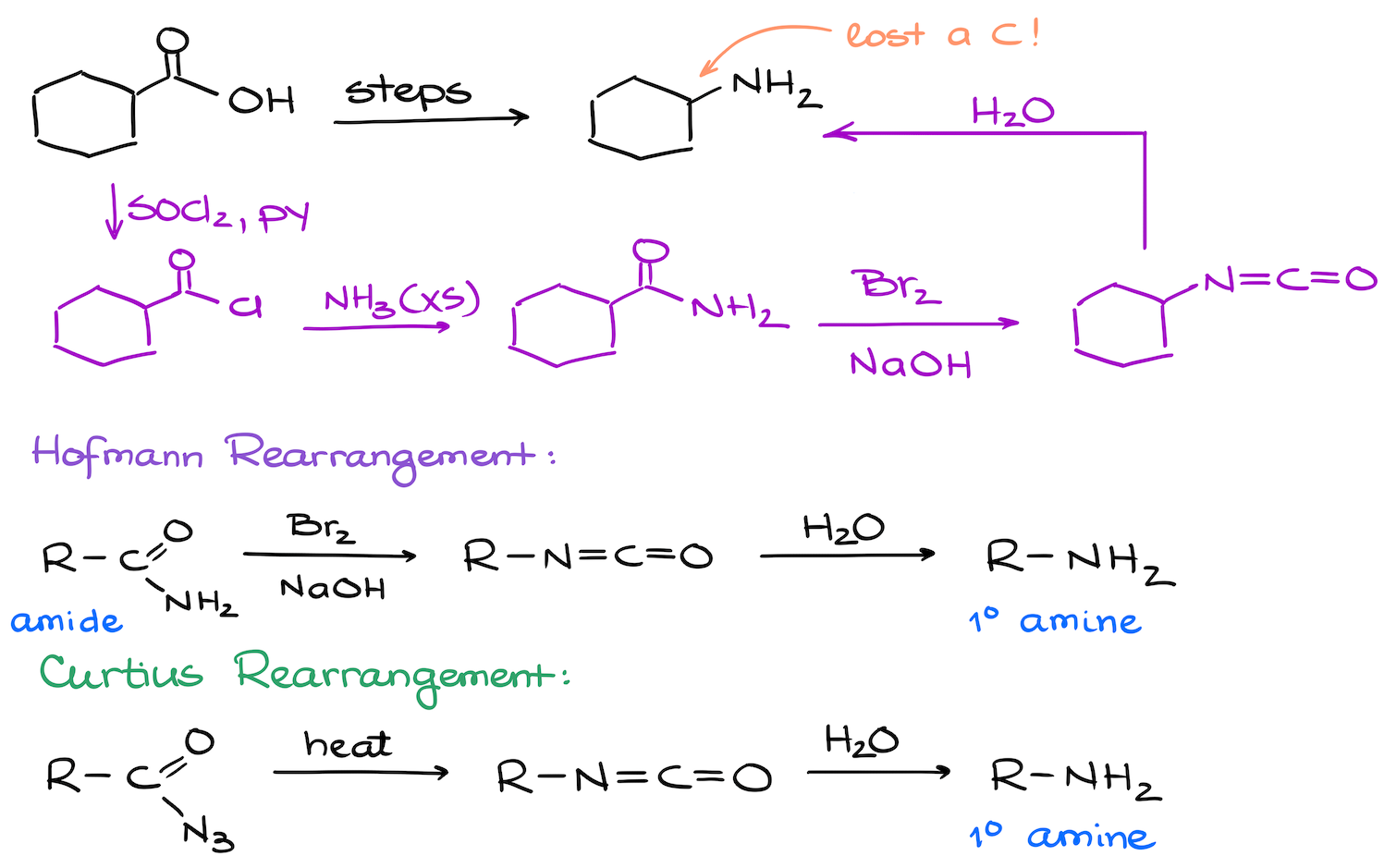
17. Synthesis of Primary Amines from Carboxylic Acids
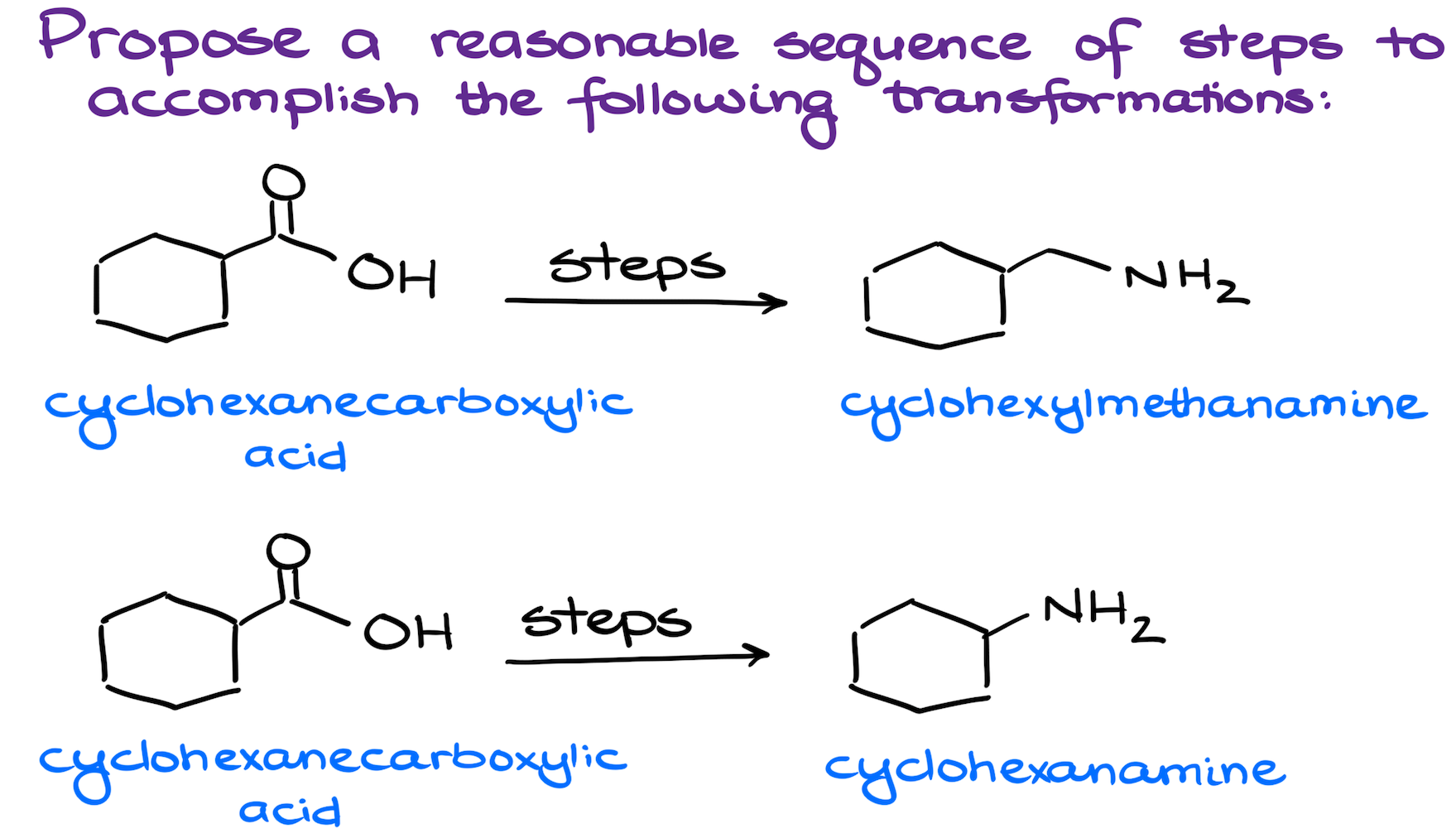
Synthesis of amines from carboxylic acids is a fairly common topic in the second semester organic chemistry. The key here is either a reduction of a carboxylic acid derivative, or Hofmann or Curtius rearrangements.
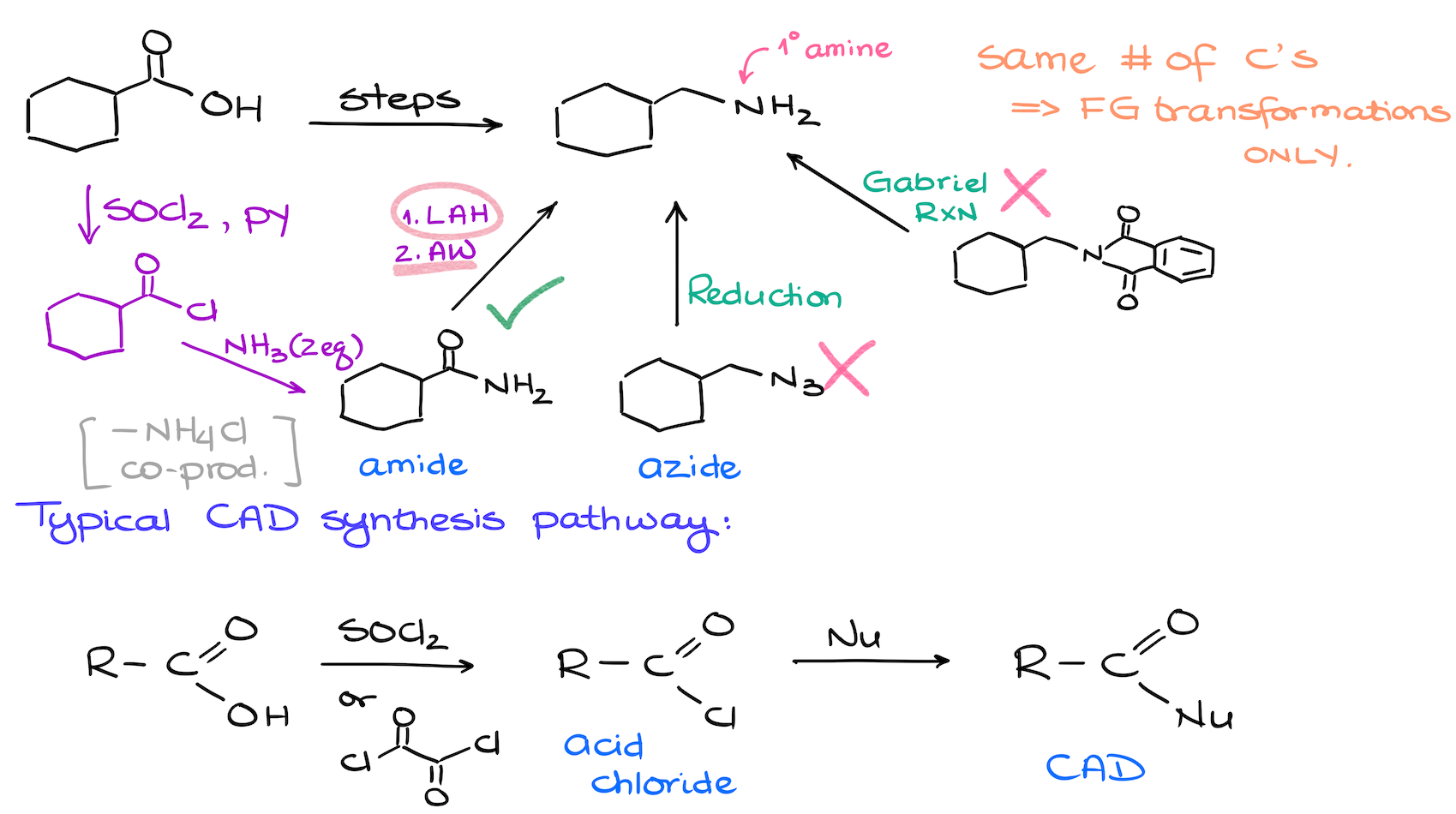
In the first synthesis we’re preserving the number of carbons we have in our molecule. This means that we’re going with the reduction of a carboxylic acid derivative ﹣ an amide in this case.
The second synthesis is a bit trickier as we’re losing one carbon. This is a hallmark of either Hofmann of Curtius rearrangements. So, our task here is to figure out how to make the appropriate intermediates for the final step.