11. Adding a Functional Group Next to a Carbonyl Challenge
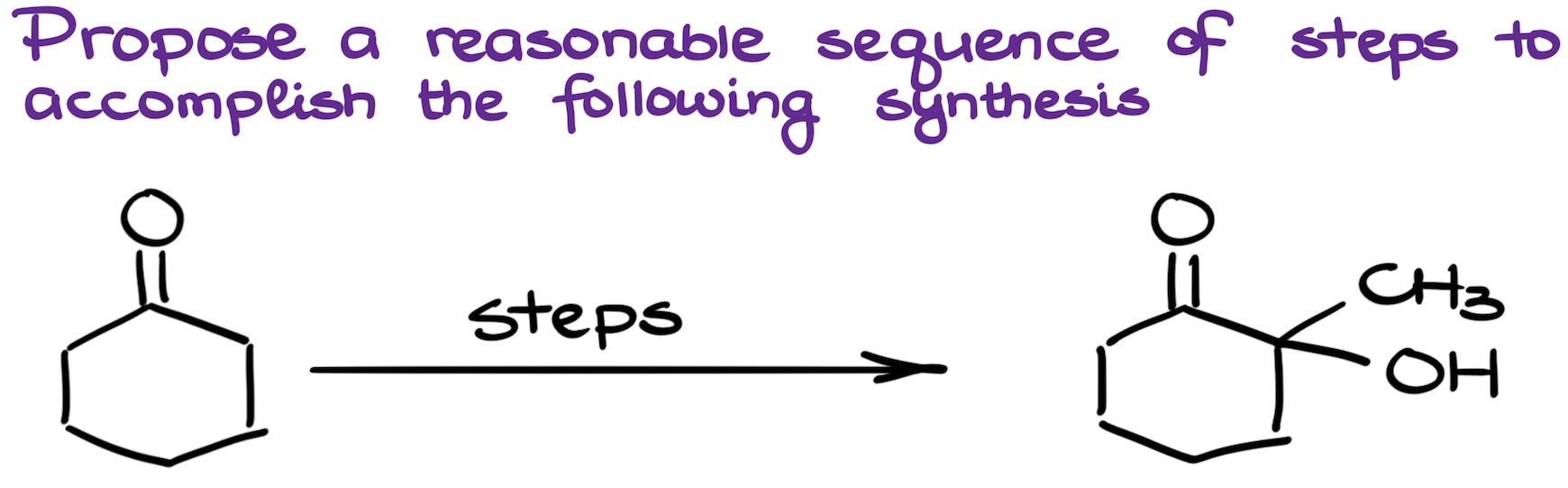
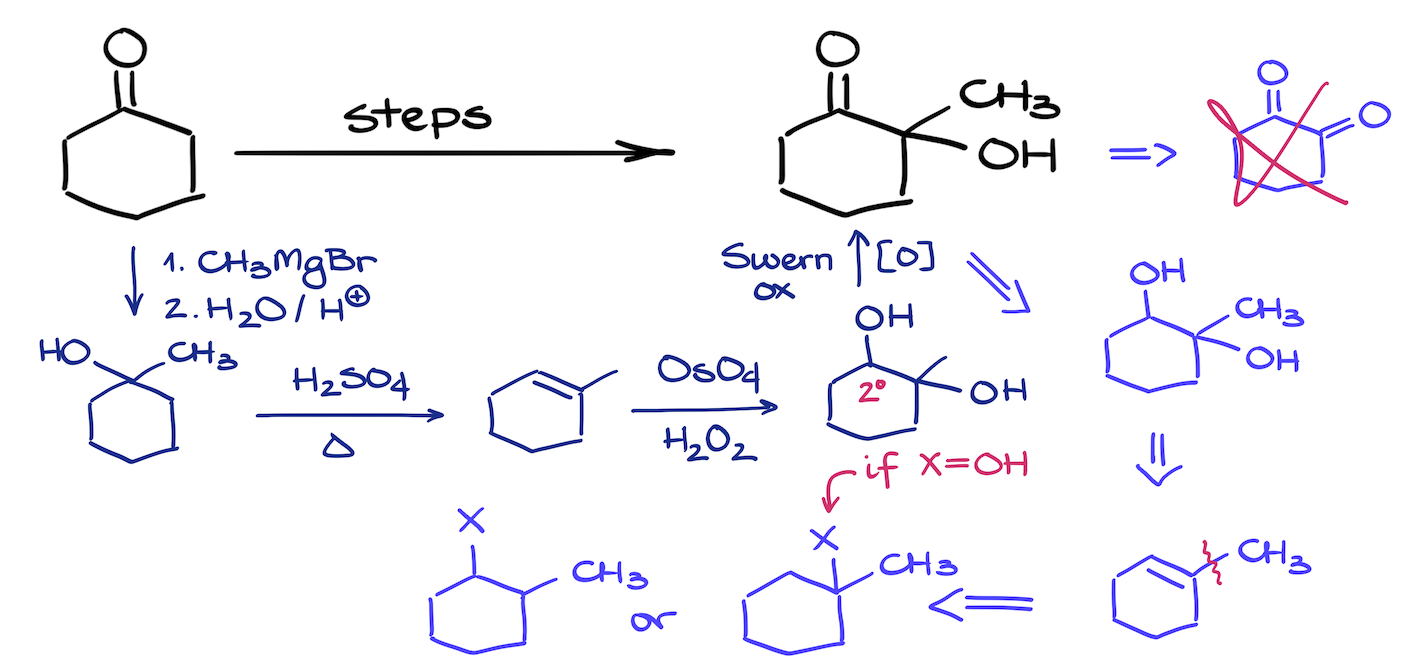
This synthesis challenge might seem like a quick work for the enolate chemistry. However, it is a bit more challenging than that. Also, if a problem like this one pops up early in the course, students would be most likely unfamiliar with the chemistry of enols and enolates. So, what are we going to do in this case?
We’ll have to add functional groups using the methods that we know (assuming we’re currently in the first semester of a typical organic chemistry course). The trick here is to remember that aldehydes and ketones are often synthesized via an oxidation reaction. But if we have a 3° alcohol, we won’t be able to oxidize that group. This means, that if we make a diol where one of the alcohols is tertiary, we can simply perform an oxidation reaction and end up with the target molecule without having to use any advanced synthetic strategies.
Reactions that you’ll see in this synthesis are: