Mechanism Challenge: Epoxide Formation
In this post, I want to go over a really fun mechanism challenge. When I posted this mechanism on my Instagram, a lot of you commented and messaged me with the questions regarding this mechanism. Specifically, many of you were very curious how the groups seemingly “jumped” around. Well, let’s look at this mechanism and figure it out together!
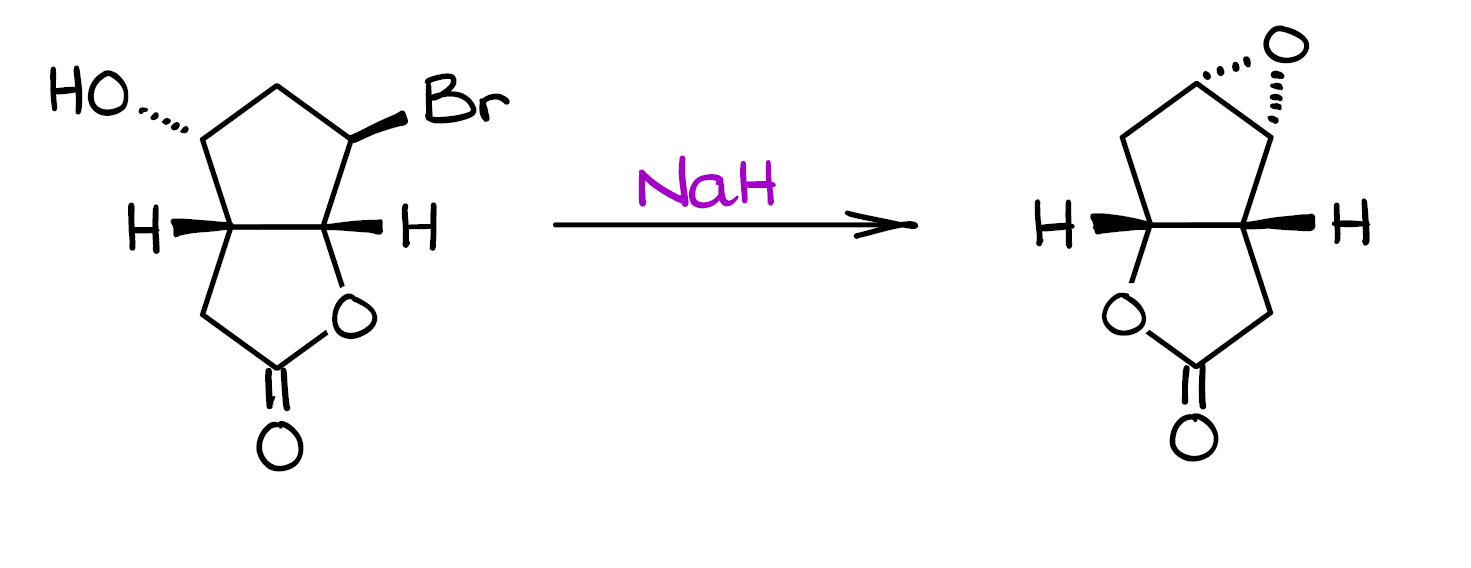
Before we go into the details of this mechanism, I suggest you pause this post and give it a try. Then, once you have something worked out, you can see how your ideas match to what’s happening in this reaction.
Step 1: Acid-Base Step
Ok. So, the first step in this mechanism is going to be an acid-base reaction. How do I know that? Sodium hydride is an extraordinarily strong base and it generally does one thing—it deprotonates acidic hydrogens. The hydride in NaH is not too nucleophilic, so we don’t expect anything but the acid-base reaction or a proton transfer, if you like.
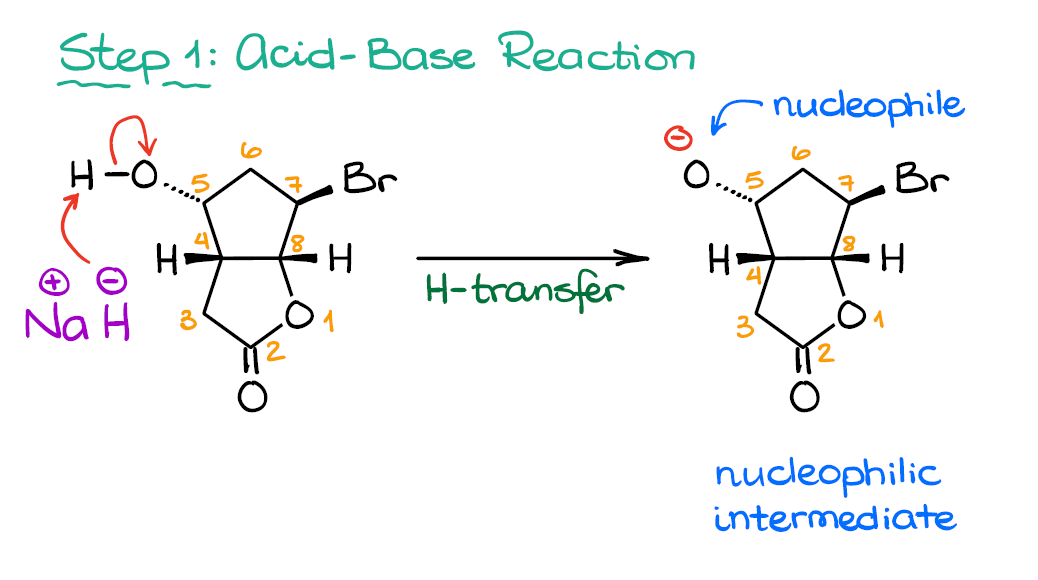
The most acidic proton in this molecule is the one on the -OH group on the 5th carbon. While protons on atom #3 are somewhat acidic as well, their pKa is roughly 21, while the one on the Alcohol is closer to 17. Thus, with 10,000 times difference in acidity, we don’t have much of a competition here. And once we pull that proton off, we get a nucleophilic intermediate which can react further.
3D Structure of the Starting Material
Here’s something I want to point out before we go any further. Here I have the structure of the starting material that I’ve made with my molecular model kit. This structure nicely illustrates the proximity of the -OH group which becomes the negatively charged O-nucleophile and the two potential electrophiles: the carbonyl of the ester (C#2) and the carbon with bromine (C#7). Both of those electrophiles are viable targets for our nucleophile. So, in theory, we can get two different products.
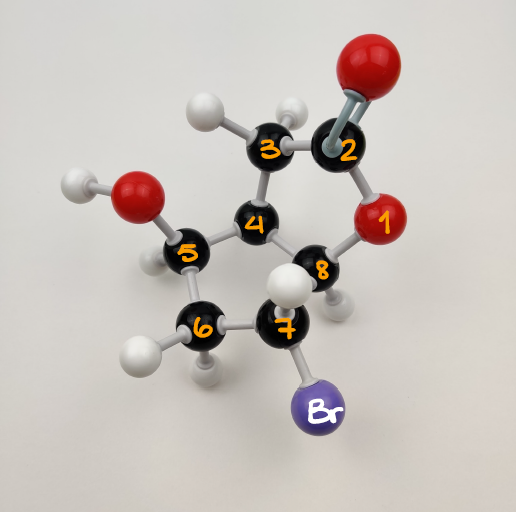
I had to flip the molecule upside down to make it a little easier to see the distances and the angles between the atoms. I’ll be keeping consistent numbering of the atoms as well throughout this whole post to make it easier to reference which atoms go where in the final molecule.
Option 1: The Nucleophilic Attack on C7
Let’s look at the first option. Here we have a nucleophilic attack of the alkoxide on the C7 electrophile.
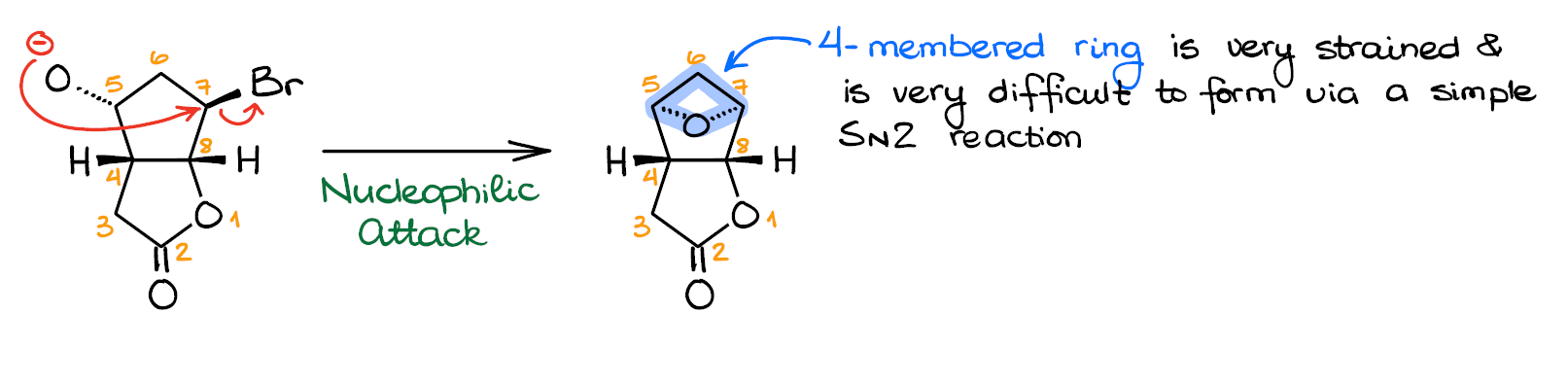
This would be your typical SN2 reaction in which you displace the leaving group (Br) with an incoming nucleophile O-minus. This gives us the product featuring the oxetane ring.
And this is where we have a problem: the 4-membered ring is rather unstable. You may recall that epoxides, which are the 3-membered rings with oxygen, are even less stable, yet they form easily in reactions like that. And you would be absolutely correct. However, in the similar reaction where the intramolecular SN2 reaction yields an epoxide, we have a proximity effect in place. Essentially, the proximity of the nucleophile and an electrophile is the driving force behind the epoxide formation. But if we don’t have the reactive centers on the adjacent atoms, then there’s no proximity effect, and there’s no oxetane formation.
Making a 4-membered ring is quite a challenge. We only know a handful of methods yielding those, and most of them give poor results anyways.
So, in conclusion, we can say, that the oxetane formation here is unlikely.
Option 2: Attack on the Ester
Now, how about the reaction with the carbonyl?
The nucleophile and the electrophile are very close to each other as well.
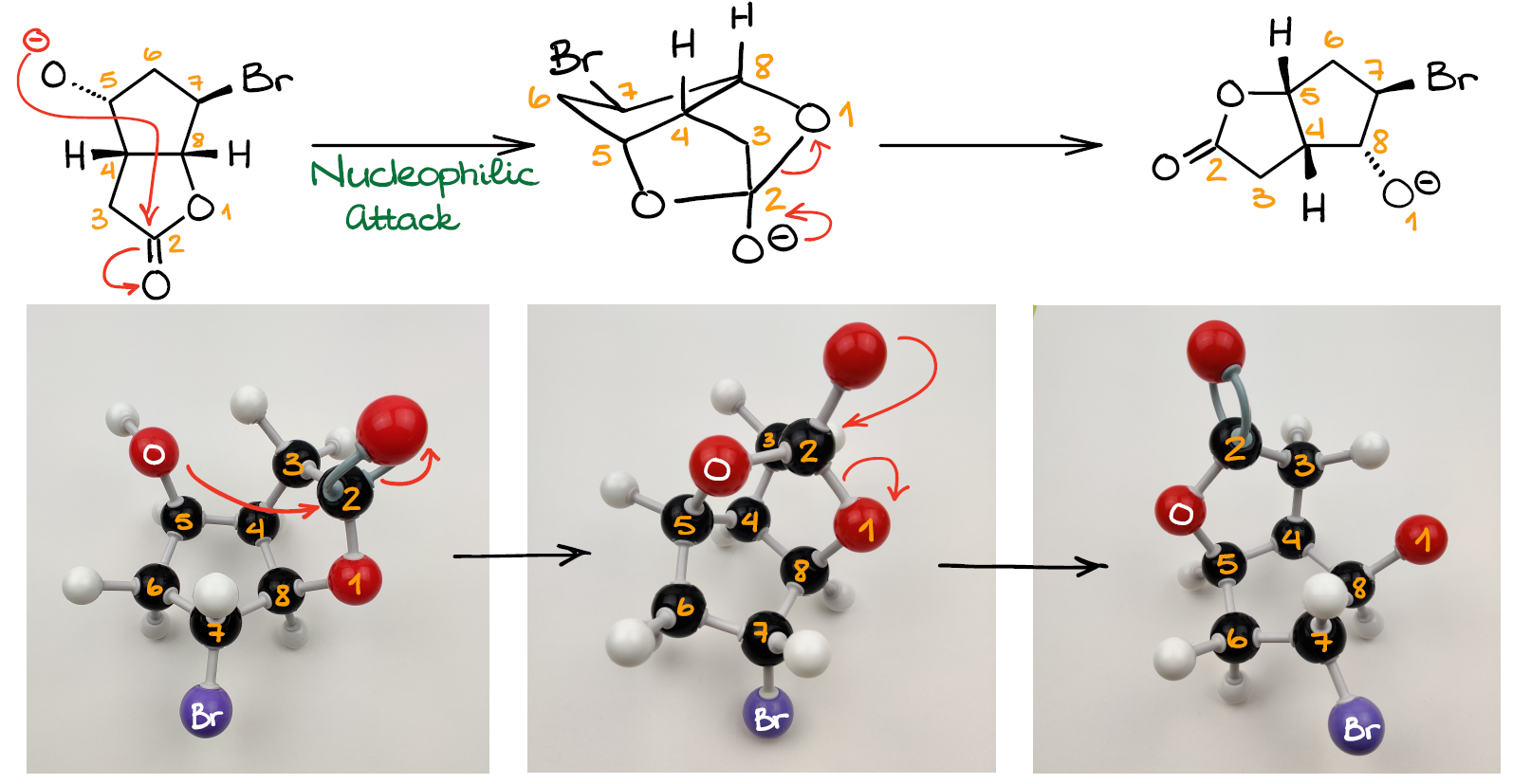
And what’s more important in this case, we’re not going to have to deal with the highly unstable 4-membered ring any longer! The intermediate here features a caged tricyclic system with 5- and 7-membered rings, all of which are stable.
Once we form this tetrahedral intermediate, we are going to go through the typical for an acyl transfer reaction—the leaving group dissociation.
This yields another pair of an ester and an alkoxide. Notice how this new molecule is structurally very similar to the original starting material. However, in this case, all groups have “shifted” around a bit. This is, perhaps, the trickiest part of this entire reaction—the starting material and the product both feature the bicyclic lactone backbone making them seemingly similar. In reality though, you can now see that we have changed the atom connectivity in the backbone itself.
Formation of the Epoxide
And finally, we can have the SN2 reaction between the new nucleophile that we just made and C7 with the leaving group (Br). This gives us our final product—epoxide. And this concludes this entire mechanism.
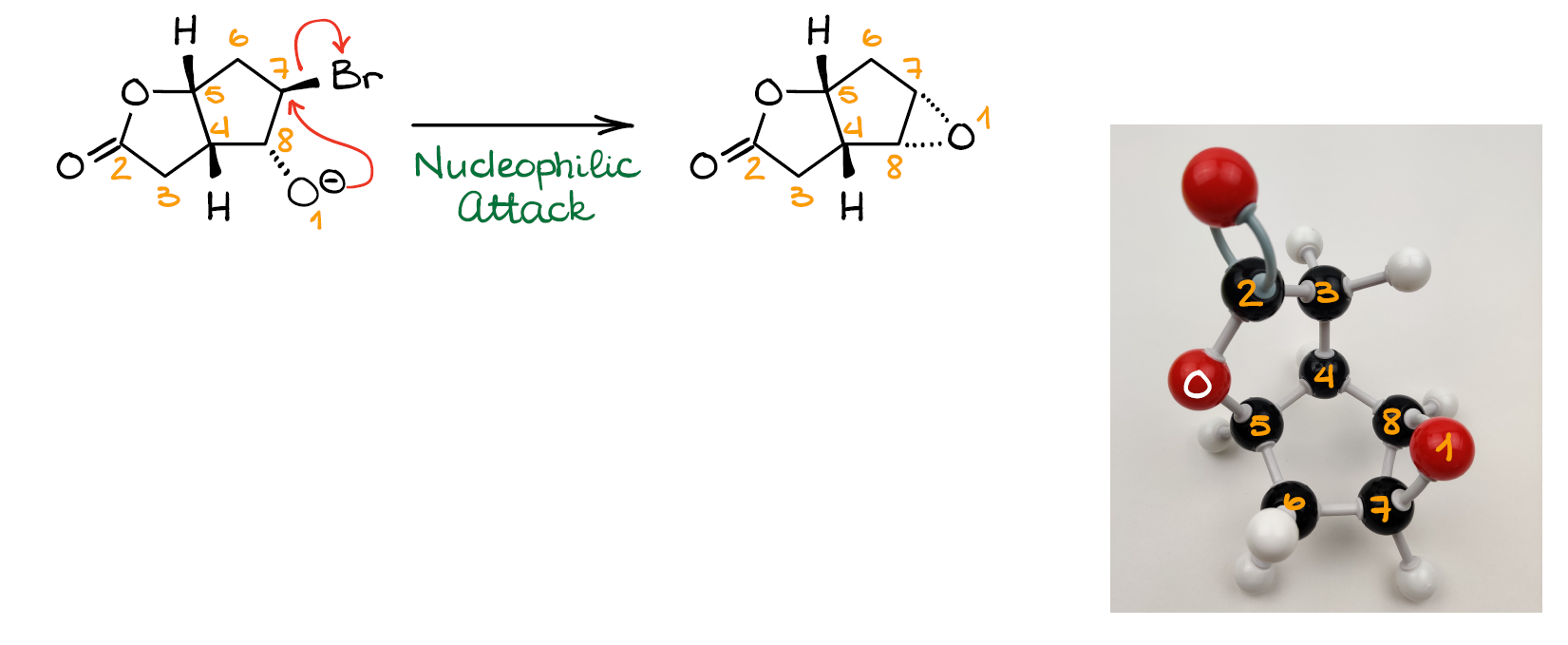
So, as you can see, nothing magically jumps around or changes locations without any obvious reason. The tricky part here was to catch that bicyclic structure rearrangement, which made the reactive centers appear next to each other and facilitate the formation of the epoxide product.
Hope you folks enjoyed this little mechanism challenge here and learned some new ways of looking at and analyzing your reactions.
