Hybridization and VSEPR Theory
In this tutorial we are going to go over a couple of fundamental topics important for any organic chemistry student. The VSEPR theory helps us to predict the 3D shapes of the molecules. As organic chemistry is a 3D-based science, the shapes of molecules are extremely important for the complete understanding of organic reactions and properties of molecules.
The concept of hybridization helps us understand the bonding in organic molecules. While hybridization might be a simplistic approach, it is sufficient enough for our purposes. You’re going to be referring to hybridization quite often, so it’s definitely one of the must-know topics.
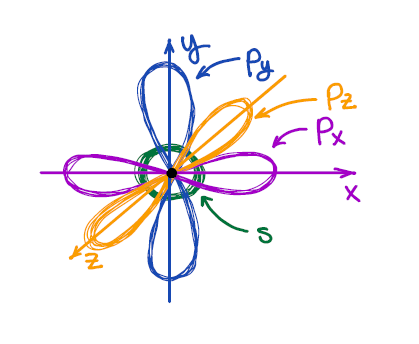
We all know from general chemistry that the s-orbital is spherical, and p-orbitals are dumbbell-looking orbitals oriented along the x, y, and z axes of the Cartesian system. We also know that VSEPR describes the 3D shape of the second period elements reasonably well.
And here we have a problem: the atomic orbitals are at 90° to each other, while the VSEPR theory predicts the 3D structure of, say, methane (CH4) to be tetrahedral with bond angles around 109.5°. So, how can we have 109.5° bond angles made by the orbitals which are at 90° to each other? 🤯🤷♂️🤔
In 1931 the twice Nobel Laureate Linus Pauling proposed the model of “mixing” the orbitals or “hybridizing” them to account for the observed bonding pattern. Pauling proposed sort of a combination of the orbitals giving you an orbital that has partial characters. Not a complete s- or a p-orbital, but rather something with a partial s- and partial p-character.
Let me emphasize one more time that hybridization is a mathematical model. There’s no actual “process” that happens to orbitals that causes the hybridization. The atomic orbitals don’t actually change before going into the bonding with other atoms. Hybridization is a mathematical model that describes how the atomic orbitals would’ve looked like based on the observable molecular orbitals.
Formation of the Hybridized Orbitals
Ok, now when we know that hybridization is a model and not an actual process, let’s look at how this “process” happens. 🤣 Each bond takes 2 electrons to complete. If we look at the carbon atom atomic orbitals, we’ll see the 2 electrons on the 2s and 2 electrons on the 2p shells. This would only allow carbon to make 2 bonds since it only has 2 unpaired electrons. To make four bonds, carbon would have to “decouple” its s-electrons onto the p shell.
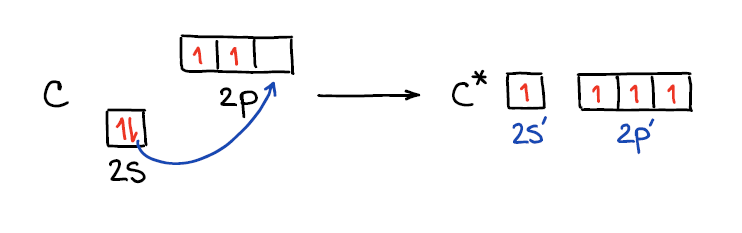
Rearranging the electrons in an atom in this way also makes the orbitals closer in energy making them virtually degenerate. This allows for easier “mixing” or hybridization as we know it. Thus, when we mix those orbitals together we end up with a set of “hybrids” and any leftovers that were not hybridized.
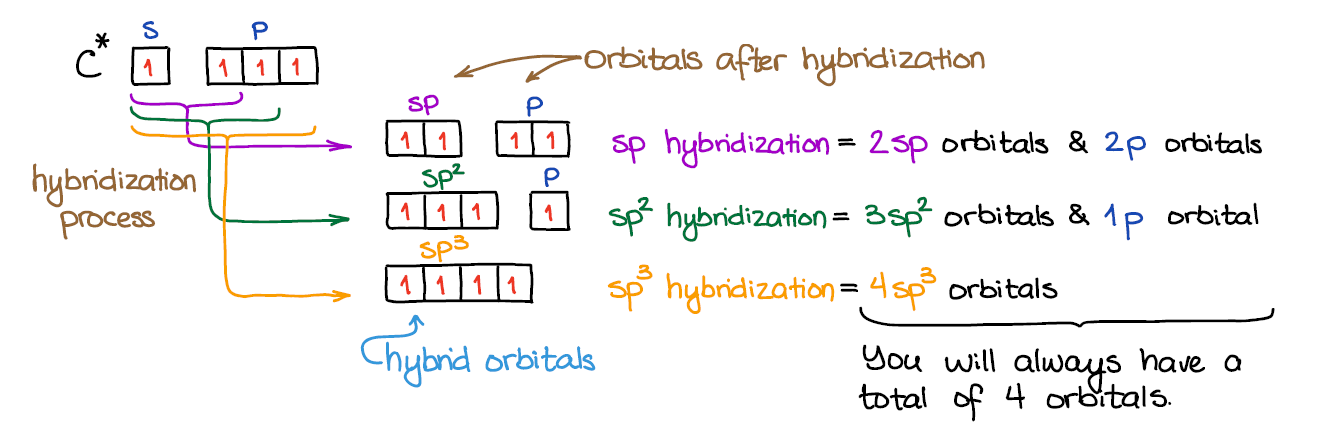
An important thing to remember: # of AO’s = # of MO. So, when we mix the atomic orbitals to make the hybrids, we will end up with the exactly the same number of the the orbitals when we’re done. Thus, by mixing 4 orbitals (one s and three p), we’ll always get 4 molecular orbitals (hybrids or not).
sp3 Hybridization
Mixing an s-orbital and three p-orbitals gives four sp3 orbitals.
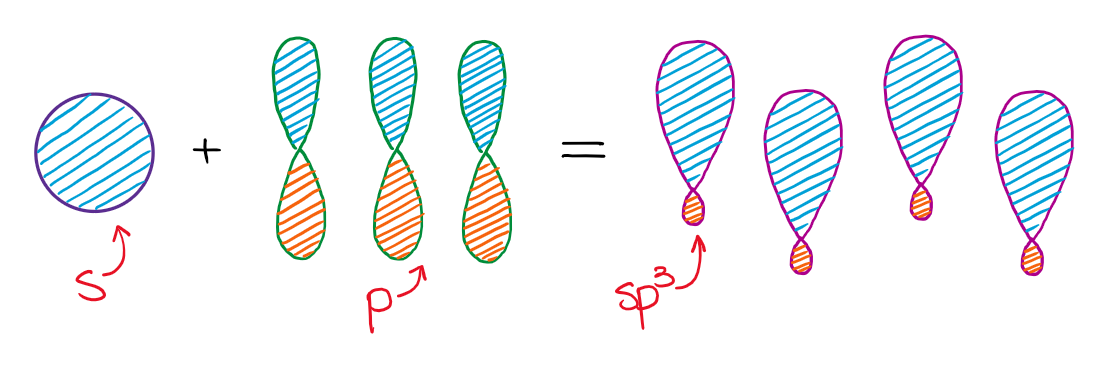
The resulting four hybrid sp3 orbitals are all degenerate in energy, meaning they are all the same. Also, according to VSEPR theory, those orbitals need to be as symmetric around each other as possible. This gives a tetrahedral structure with bond angles around 109.5°.
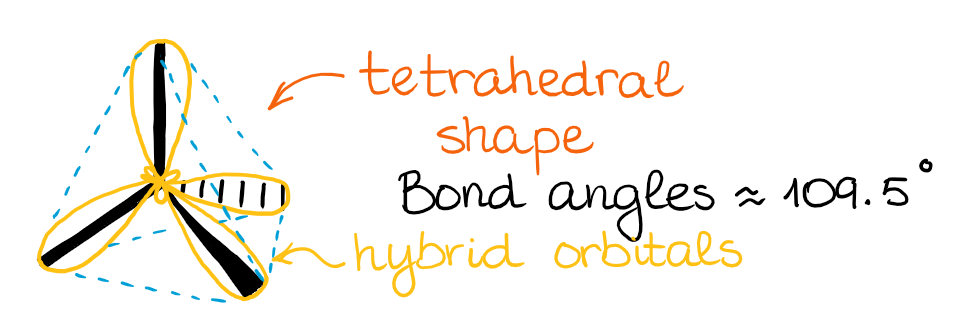
When those hybrid orbitals make bonds, we get molecular orbitals oriented in the same direction. So, as I’ve mentioned earlier, while the hybridization and the hybrid orbitals might be the mathematical model, it does help us predict and illustrate the actual molecular orbitals in the molecule. BTW, the molecular orbital theory (MOT) is a mathematical model as well. 🤣 However, we can perform calculations using the MOT to predict the electron densities around the molecule congruent with the real physical observations.
sp2 Hybridization
When we mix one s-orbital and two p-orbitals, we get three sp2 hybridized orbitals. You’ll also have one leftover p-orbital that didn’t participate in the hybridization.
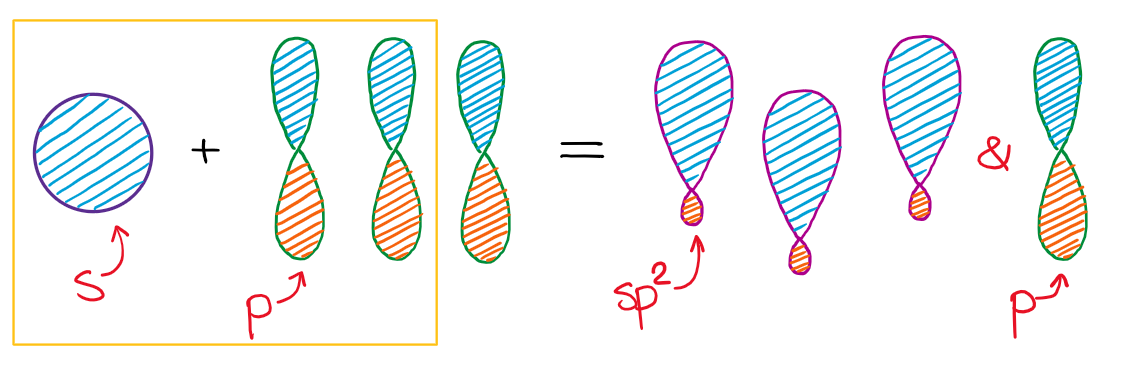
This hybridization gives you the trigonal planar geometry around the central atom with the p-orbital sticking in the up and down vertical direction.
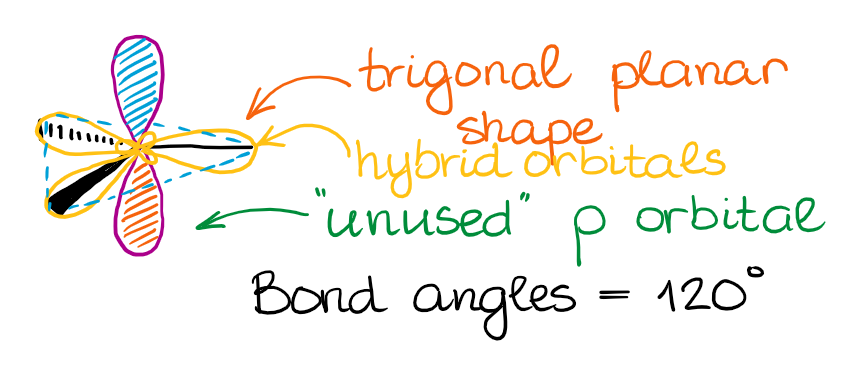
The “unused” p-orbital can make a π-bond or to participate in a complex resonance conjugation.
sp Hybridization
In the case of the sp hybridization, only one s- and one p-orbital are mixed together to make hybrids. This leaves two unused p-orbitals.
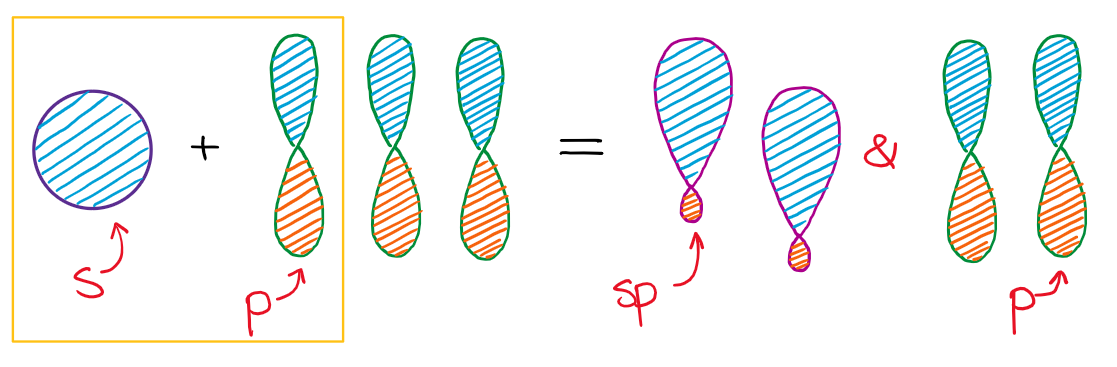
The unused sp orbitals force the structure to have a linear 3D geometry.
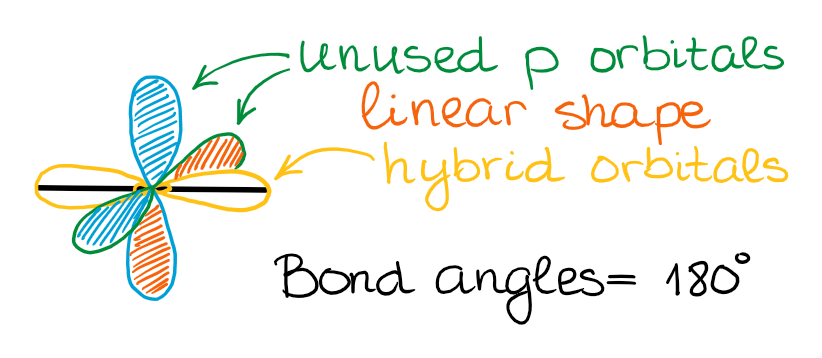
The unused p-orbitals can make two double bonds, a triple bond, or potentially participate in resonance with other orbitals.
How Do We Determine Hybridization?
The easiest way to determine hybridization is to with the VSEPR theory and determine the number of electron groups around your central atom. To put it plain, I can summarize the hybridizations in the following picture:
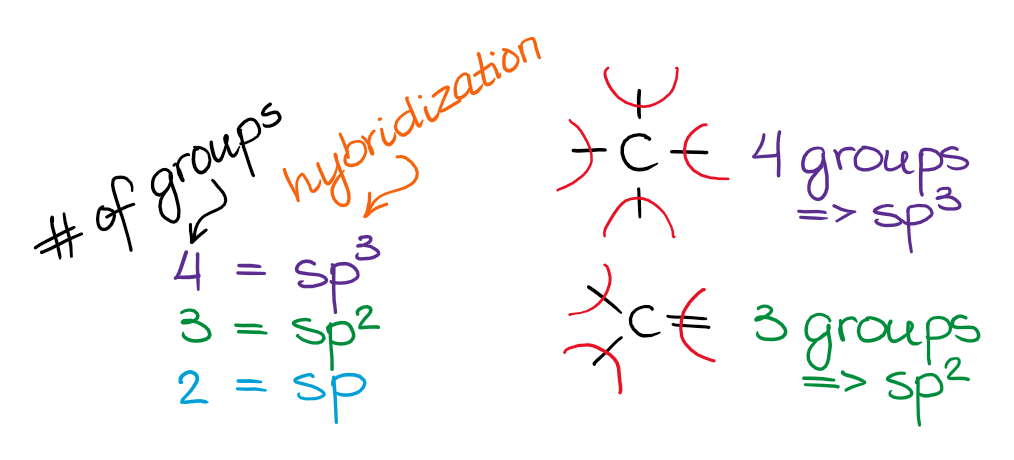
So, the 3 groups around the central atom gives you the sp3 hybridization, the three groups gives you sp2 hybridization, and the two groups yield the sp-hybridized species. Also remember, we do count the the spare electron pairs as the electron groups too!
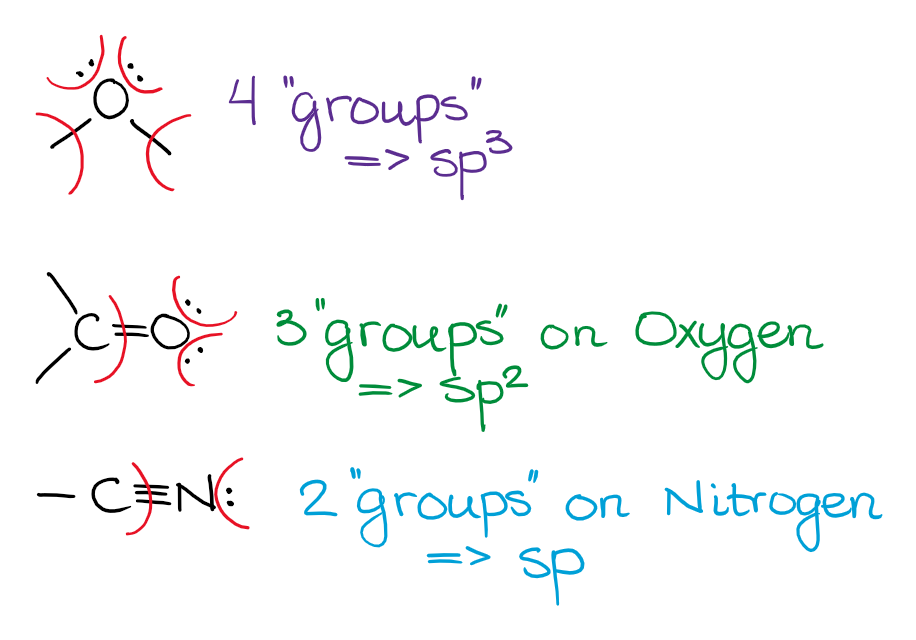
Unless the electron pair is next to a double or a triple bond (or an empty p-orbital), the electron pair will be on the hybrid orbital and not the p-orbital. While that is not 100% true in reality, that’s the way we treat it within the scope of a typical organic chemistry class, so we’ll stick with it too.
Hybridization of Atoms with Electron Pairs next to Double or Triple Bonds
I’ve mentioned above, that a double or a triple bond next to an electron pair matters. When you have an electron pair next to a p-orbital or a π-bond, there’s a resonance between those.
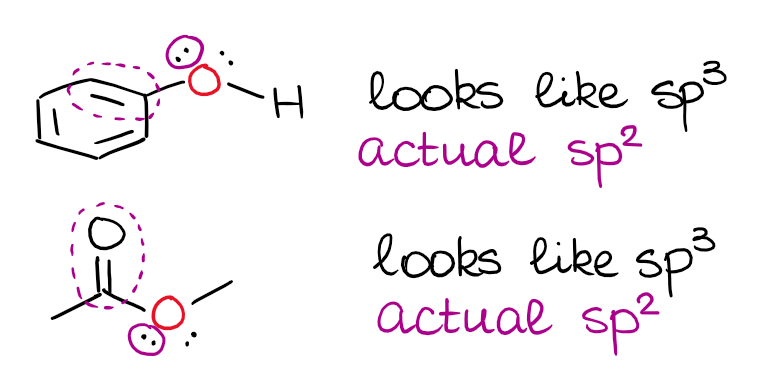
This is a very typical “trick question” on the exam, so you wanna keep this in mind. It’s also important to remember that the electron pair has to be physically able to align with the p-orbital or a π-bond for this to happen. So, the isolated electron pairs will still be sitting on the hybrid orbitals even when they are next to double bonds.

Spotting the isolated electron pairs can be a little tricky, so you may wanna do some practice to master this skill. There is a quick rule of thumb you can use. If you have an electron pair on the atom that already has a double bond, chances are, it’s going to be isolated. It’s not a 100% foolproof trick, but it works for cyclic structures.
VSEPR Theory
VSEPR stands for Valence Shell Electron Pair Repulsion Theory.
We use VSEPR to predict the 3D shapes of the molecules made by the 2nd period elements. The main focus in this topic is going to be on the carbon (C), nitrogen (O), and oxygen (O). Those three elements make the “core” of the organic molecules, so you’re going to be working with those most of the time.
How Do We Apply the VSEPR Theory?
The premise of the VSEPR is the idea that the electron pairs & bonds will distribute themselves as far from each other as possible around the central atom. Think about a bunch of balloons tied to a single point. That would be a pretty accurate description of the approach.
While there are quite a few electronic domains and, thus, 3D shapes, we only focus on three shapes in organic chemistry.
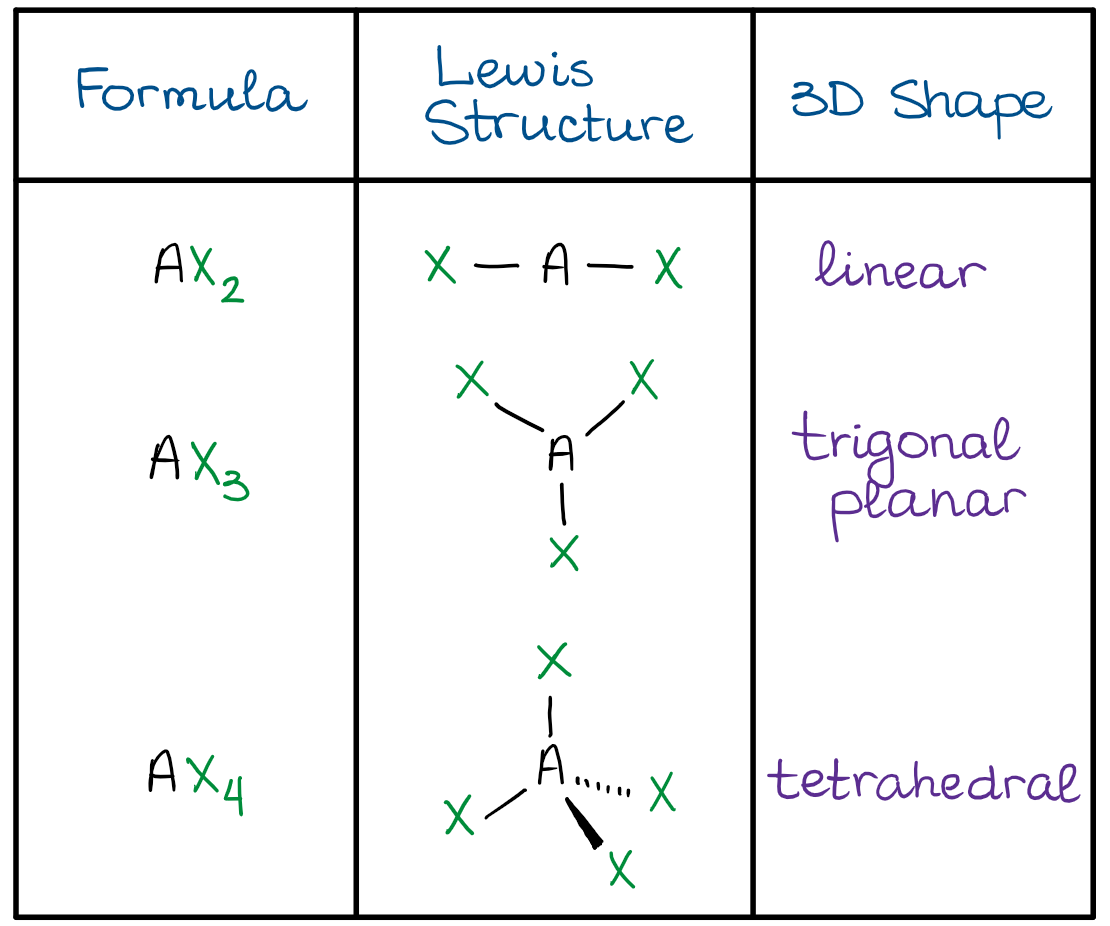
- The linear shape means that all three atoms are making a linear string of 3 atoms in a line. Thus, the X-A-X bond angle is 180º.
- The trigonal planar shape has a central atom (A) in the middle of the molecule, while the rest of the groups are making a perfect triangle around it. This gives a X-A-X bond angle of 120º.
- The tetrahedral shape resembles a trigonal pyramid with all sides being perfect triangles. The X-A-X bond angle is a little more difficult to calculate, but it is approximately 109.5º.
The most important domains for us are going to be the AX3 and AX4. Those are the two most common shapes we’ll see in organic molecules.
What is the Difference Between the Electronic Domain and Shape?
The difference comes when we have spare (non-bonding) electron pairs instead of the groups sitting around the central atom. The electron pairs are “invisible” for the purposes of the shape. However, since they are still there, they do influence the shape and thus are important to remember. So, how does the VSEPR theory treats this difference?
The Tetrahedral Domain
In the tetrahedral domain, there are four “things” attached to the central atom. Those “things” can be either groups or electron pairs. Depending on how many electron pairs we have, we’ll end up with the following shapes.
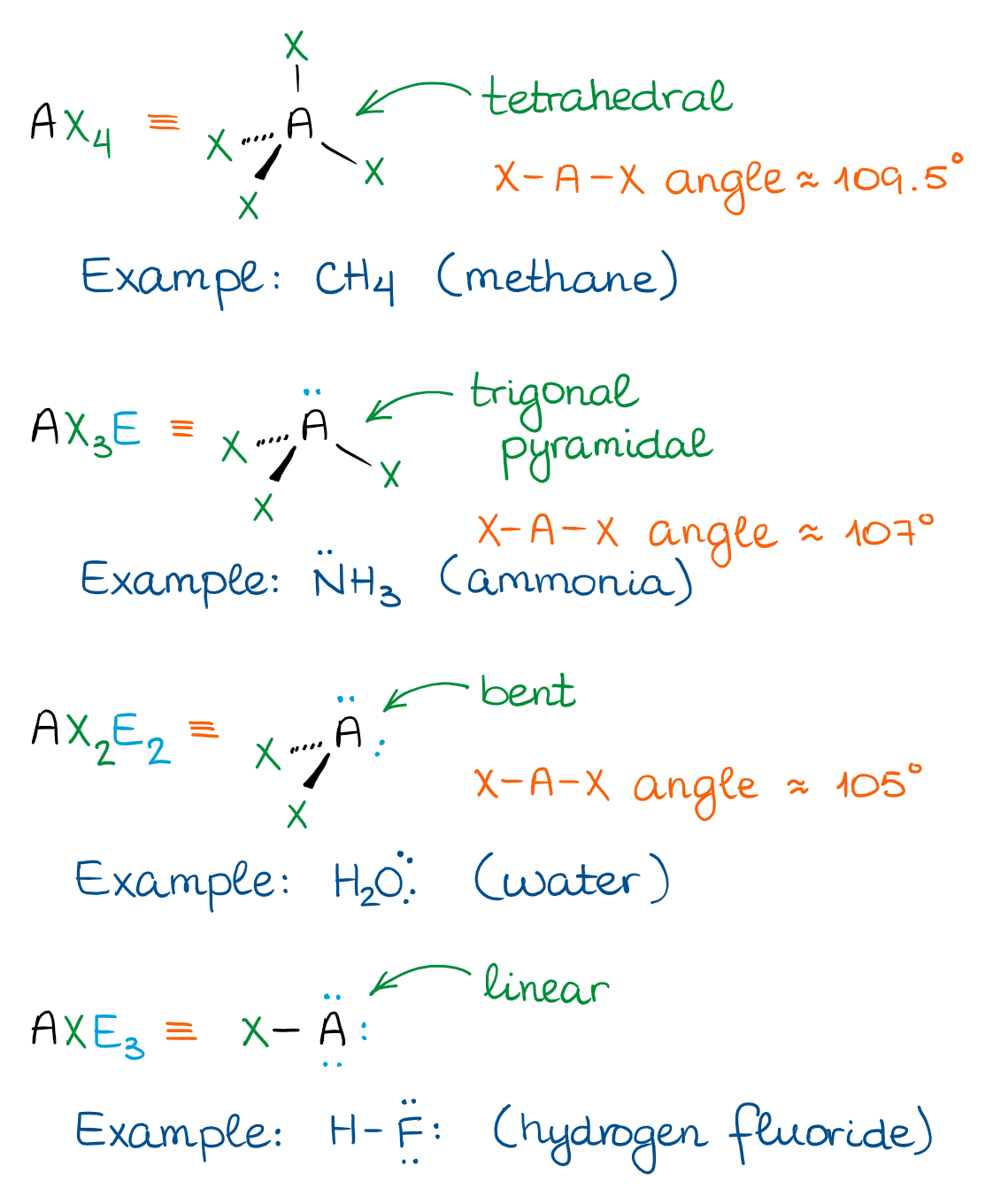
The tetrahedral domain is the hallmark of the molecules with single bonds. Those are also called “saturated” meaning that those molecules cannot add any hydrogens. We’ll talk about those addition reactions later on, so don’t fuss about the name for the moment 😉
The Trigonal Planar Domain
When the central atom is connected to one of the groups by a double bond or has an empty p-orbital, we get the trigonal planar domain. There are less shapes associated with this domain than with the tetrahedral though, so it makes it easier to remember.
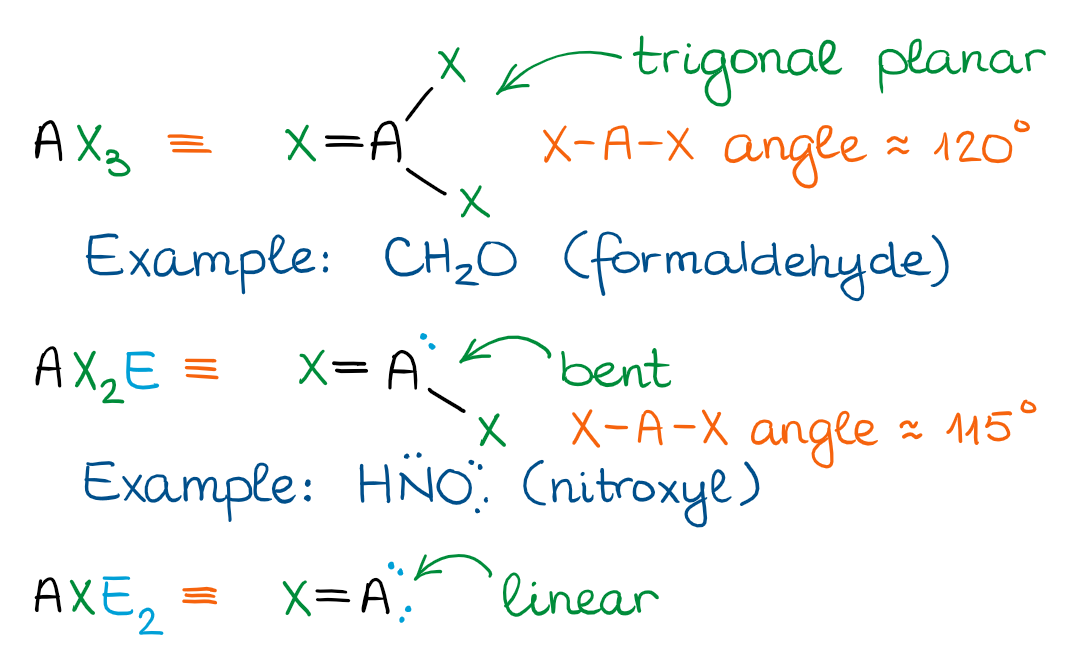
The figure above shows only the cases with the double bonds. We’ll discuss the examples with empty orbitals (such as carbocations) later in this course. Structures with empty orbitals are very unstable, so we’re only going to see those as highly reactive compounds or intermediates in reactions.
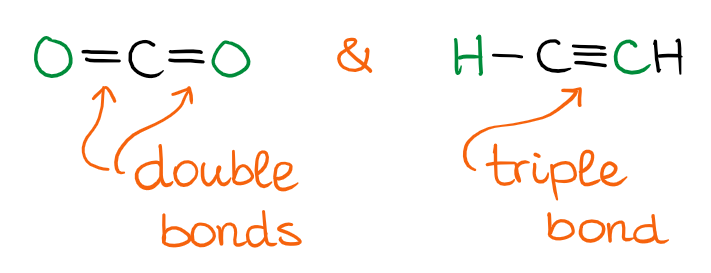
The Linear Domain
When you have two groups attached to the central atom by double bonds, or if you have a triple bond, you’ll have a linear domain. As linear molecules are very simple, there’s not much to discuss shape-wise here. They are, well, linear. 😹
Limitations of VSEPR Theory
Larger atoms distort the structures more than the small ones. So, when you have a molecule with atoms from the 3rd period and beyond, you’ll see significant deviations from the VSEPR shapes and bond angles. Within the scope of a typical organic chemistry course, you’ll only be responsible for estimating if the bond angles are going to be about what they are supposed to be or larger/smaller than that.
