THF (Tetrahydrofuran)
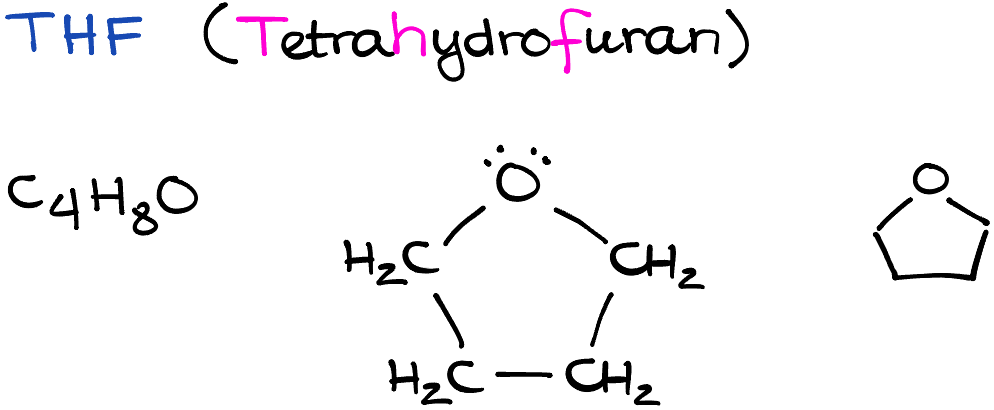
Introduction
Tetrahydrofuran, often abbreviated as THF, is a ubiquitous chemical compound in the realm of organic chemistry. Boasting a simple structure—four carbon atoms forming a ring with an oxygen atom, THF’s simplicity belies its immense importance. This polar aprotic solvent often plays a pivotal role in substitution reactions, particularly SN2 reactions. In this entry, we will delve into the physical properties of THF, its role in organic chemistry, and some interesting factoids about this seemingly ordinary yet extraordinarily essential chemical compound.
Physical Properties
THF is a colorless, volatile liquid, carrying a faint, ethereal odor reminiscent of its chemical cousin, diethyl ether. The density of this liquid is approximately 0.89 g/cm³, and it has a relatively low boiling point for an organic solvent, around 66°C (151°F), which makes it easy to remove by evaporation in laboratory applications.
One of the interesting aspects of THF is its dipole moment. Its oxygen atom forms two bonds with neighboring carbon atoms, but it also carries two lone pairs of electrons, which makes THF a polar molecule. Furthermore, THF is classified as a polar aprotic solvent because, although it has polar bonds, it does not have any hydrogen atoms directly attached to these polar bonds, preventing it from forming hydrogen bonds with solutes.
Role in Organic Chemistry
THF is renowned in organic chemistry for its role as a solvent, especially in substitution reactions, such as the SN2 mechanism. The SN2 reaction is a type of substitution reaction where a nucleophile (“nucleus-loving”) atom or molecule displaces a group (the leaving group) in a molecule. The polar nature of THF makes it an excellent medium for such reactions, as it can stabilize ions—a vital condition for the SN2 reaction to take place.
THF is particularly adept at solvating cations, thanks to the oxygen atom’s lone pairs of electrons. This ability comes in handy when performing reactions with organolithium or Grignard reagents, where it can help stabilize the highly reactive lithium or magnesium cations. Despite being polar, the lack of acidic hydrogens in THF means it doesn’t react with these strong bases, making it an ideal solvent for these types of reactions.
Safety Information
The importance of safety cannot be overstated when handling tetrahydrofuran (THF). As a highly flammable compound, THF presents a significant fire risk. Direct exposure to heat, sparks, or open flames must be avoided at all times. THF vapors can form explosive mixtures with air, emphasizing the necessity of proper ventilation when working with this solvent.
Another critical safety consideration with THF is its tendency to form dangerous peroxides upon prolonged exposure to air. These peroxides can explode with shock, heat, or friction. It’s of paramount importance to store THF properly, usually under an inert atmosphere, and to check old bottles regularly for any evidence of peroxide formation. If there is a suspicion of peroxide contamination, the solvent should not be moved, and a professional should be contacted to dispose of it properly.
In terms of health effects, THF can be harmful if inhaled, ingested, or if it comes into contact with the skin. It can cause irritation to the eyes, skin, and respiratory tract. In severe cases, prolonged or repeated exposure may damage the liver and kidneys. Always use personal protective equipment, such as gloves and safety glasses, and work in a well-ventilated area when handling THF.
Lastly, the environmental impact of THF must not be ignored. THF is harmful to aquatic life, and therefore spills should be prevented from reaching waterways. Always dispose of THF waste responsibly, following local regulations and guidelines.
A Few Interesting THF Factoids
- Industrial Scale: In the realm of large-scale industrial chemistry, THF is produced from 1,4-butanediol. Impressively, in 2023, global production of THF was estimated to reach a staggering one million metric tons.
- Safety First: While THF is indispensable in the lab, it is also highly flammable and forms explosive peroxides upon exposure to air over time. Therefore, chemists must handle THF with utmost care, storing it properly and regularly checking older containers for dangerous peroxide formation.
- Beyond the Lab: Beyond its indispensable role in organic chemistry, THF also finds applications in the production of polyvinyl chloride (PVC) plastics and in pharmaceuticals as a precursor to the drug gamma-hydroxybutyric acid (GHB).
