The Grignard Reagent (RMgBr)

Grignard reagents stand as cornerstones in the domain of organic chemistry. Named after the French chemist François Auguste Victor Grignard, who was awarded the Nobel Prize for his work on them, these powerful tools enable chemists to perform a range of complex transformations.
Chemical Properties
A Grignard reagent is an organomagnesium compound, typically represented as RMgX, where ‘R’ is an alkyl or aryl group, and ‘X’ is a halide. These reagents are renowned for their strong nucleophilic characteristics and high reactivity, thanks to the polarization of the carbon-magnesium bond. The partial negative charge on the carbon atom allows it to behave as a nucleophile and attack electrophilic carbon atoms, such as those in carbonyl groups.
Applications in Organic Chemistry
The versatility of Grignard reagents is well illustrated by their wide range of applications. One of their primary uses is in the formation of alcohols from carbonyl compounds. For instance, when reacted with an aldehyde or a ketone, a Grignard reagent can yield a secondary or tertiary alcohol, respectively.
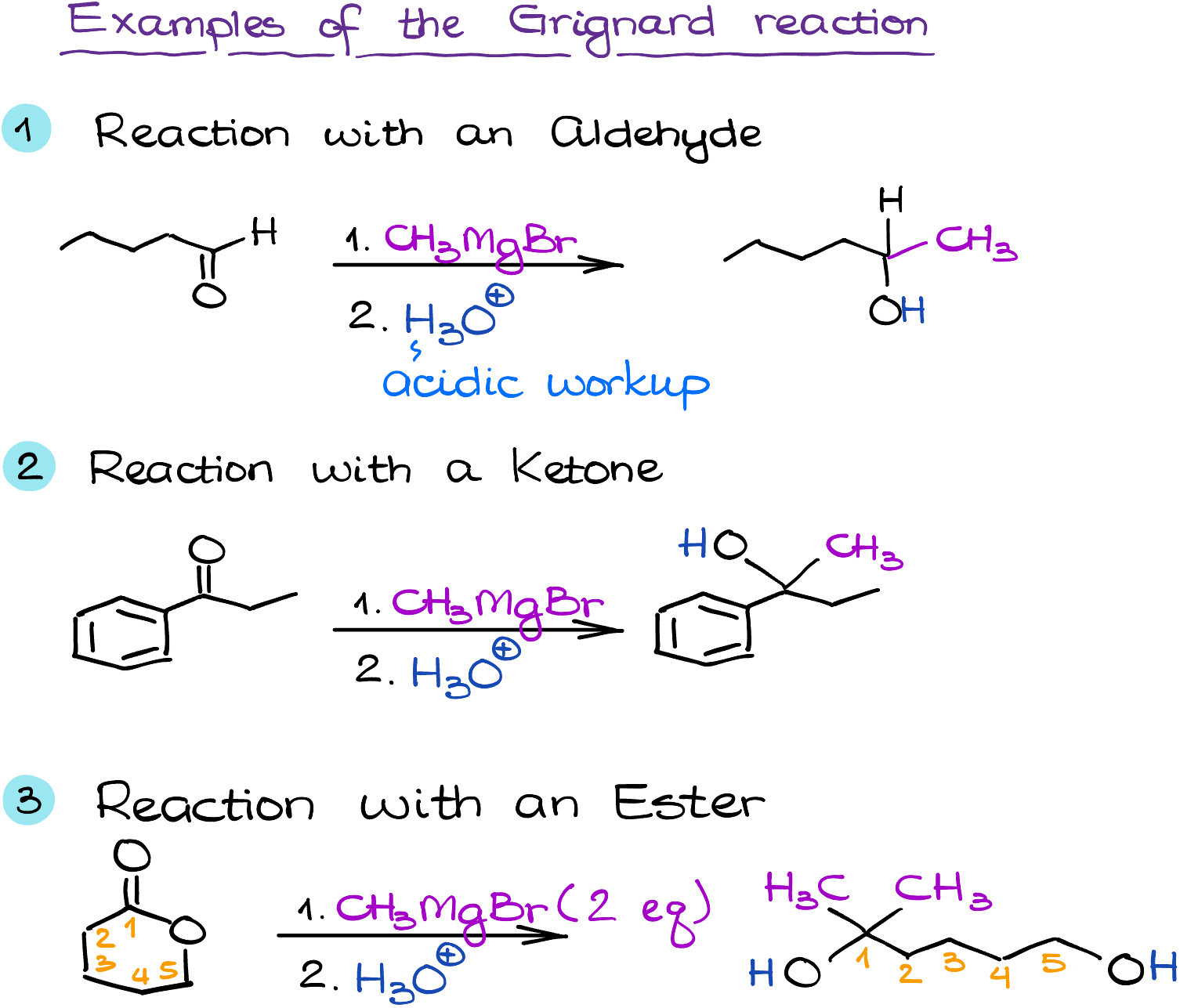
Moreover, Grignard reagents can be used in the synthesis of a plethora of other compounds, including hydrocarbons, carboxylic acids, esters, and even more complex structures like carbon-nitrogen bonds. They are critical tools in the construction of carbon-carbon bonds, the backbone of organic chemistry.
Safety Information
While Grignard reagents are incredibly useful, they should be handled with caution. They are highly reactive and can react violently with water, producing heat and flammable hydrogen gas. Therefore, the reactions involving them must be carried out under anhydrous conditions. It’s also essential to use personal protective equipment and follow safety guidelines to avoid injuries from potential chemical burns or fire hazards. Always handle Grignard reagents with respect for their reactive nature.
