Aromatic Compounds and Aromaticity
In this lesson we are going to explore the phenomenon of aromaticity and related topics.
Reactions of Aromatic Compounds (Overview)
Aromatic compounds are incredibly important in organic chemistry and targeted organic synthesis. They are also incredibly versatile in terms of the transformations they go through. Finally, they show up in a lot of natural products and medicines we use daily. Let’s look at the short list of the typical reactions of aromatic compounds that you’ll need to know for your exam. While this list is not exhaustive, these are the reactions you’re going to find in any organic chemistry textbook out there.
Halogenation of Aromatic Compounds
This is, perhaps, one of the first reaction you’re going to see in your course once you start the chapter on the reactions of aromatic compounds. This reaction is a convenient way of adding a halogen to your aromatic ring.
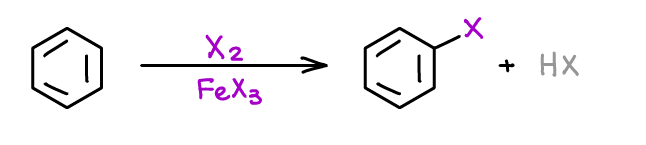
Once you have the halogen on, you can do other reactions to modify it further. Halogenation of an is often going to be one of the first reactions in your multistep synthesis involving aromatic compounds.
Friedel-Crafts Alkylation and Acylation
Friedel-Crafts is a set of two reactions both of which are looking to make a new carbon-carbon bond. In the alkylation reaction, you’re adding an alkyl group to your aromatic ring.
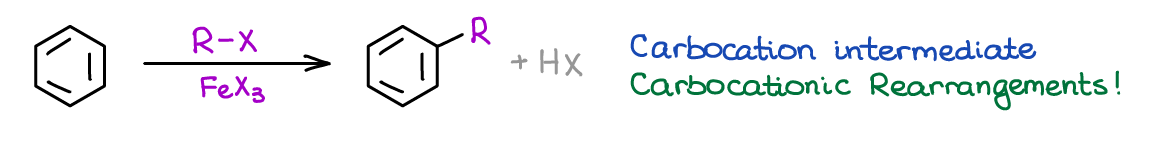
This reaction is only marginally useful as it tends to give overalkylation. On top of that, the alkyl group (-R) will easily rearrange into a more stable carbocation intermediate before attacking the aromatic ring. Alkylation also suffers from other limitations. The major limitation common between the alkylation and acylation is their sensitivity towards the nature of the aromatic compound. Friedel-Crafts doesn’t work for deactivated rings containing Electron-Withdrawing Groups (EWG’s).
Friedel-Crafts acylation, however, is a more useful reaction. First, it doesn’t give any carbocation intermediate rearrangements. Second, you’re not going to see any unwanted additional substitution reaction with it unlike in the case of alkylation.
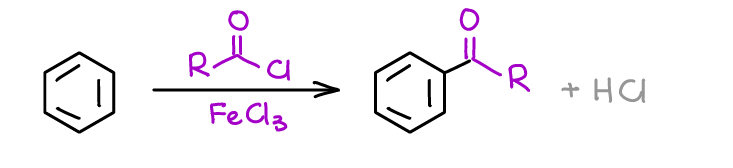
Acylation also gives you a carbonyl (a ketone in this case). This allows you to further chemically modify the C=O bond to get something else depending on your synthetic needs.
Sulfonation of Aromatic Compounds
This is a fun reaction which gives you an aromatic sulfonic acid. You may have already seen tosylic (toluenesulfonic) acid in your course (TsOH), well, that’s an example of a sulfonic acid. To make tosylic acid you just sulfonate the toluene and you got it.
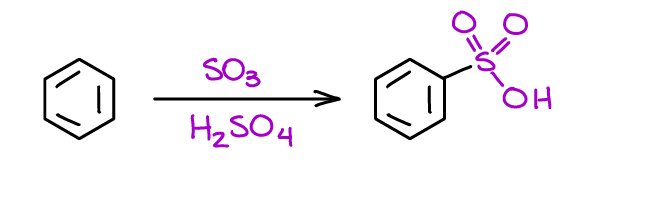
Another interesting point about this reaction is that this is the only reversible electrophilic aromatic substitution reaction. This means that you can use the sulfonic acid group as a temporary blocking group in your synthesis. That’s a very useful trick, so put it on the backburner for the future use 😉.
Nitration of Aromatic Compounds
This is another “classic” reaction of arenes. Ion this reaction, you’re going to introduce a nitro group (NO2) to your aromatic ring. By itself, the nitro group is not very useful. However, it can serve as a powerful EWG enabling other reactions like the Nucleophilic Aromatic Substitution. Or, it can be reduced to an amine, which might be difficult to obtain otherwise.
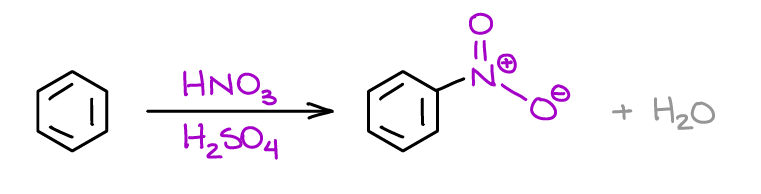
This reaction is also a very common guest on the tests, so you’ll be seeing it quite often in your course.
Birch Reduction
Birch Reduction is one of the few reactions that destroys the aromaticity in the ring. The aromatic ring is phenomenally stable, so it’s not an easy thing to do.
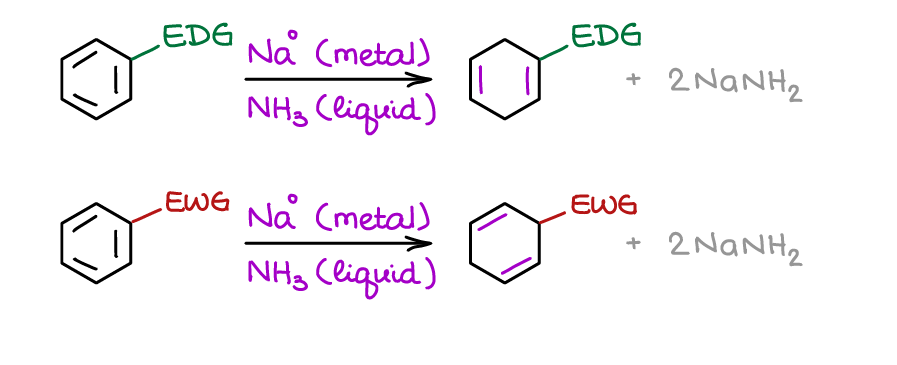
Curiously, due to the mechanism of this reaction, the nature of the groups that you already have on the aromatic ring does matter! Birch reduction has many uses in organic synthesis, so, depending on how creative your instructor is, you may be seeing it a lot or not at all 😂.
Nucleophilic Aromatic Substitution
Up until this point, all substitution reactions we’ve seen were the Electrophilic Aromatic Substitutions. In those reactions the electrophile attacked your aromatic ring and you had the -H as a “leaving group” of a sort. In the nucleophilic aromatic substitution, you’re going to have a nucleophile attacking the aromatic ring and doing the substitution. You also do have an actual “typical” leaving group in the NAS reactions.
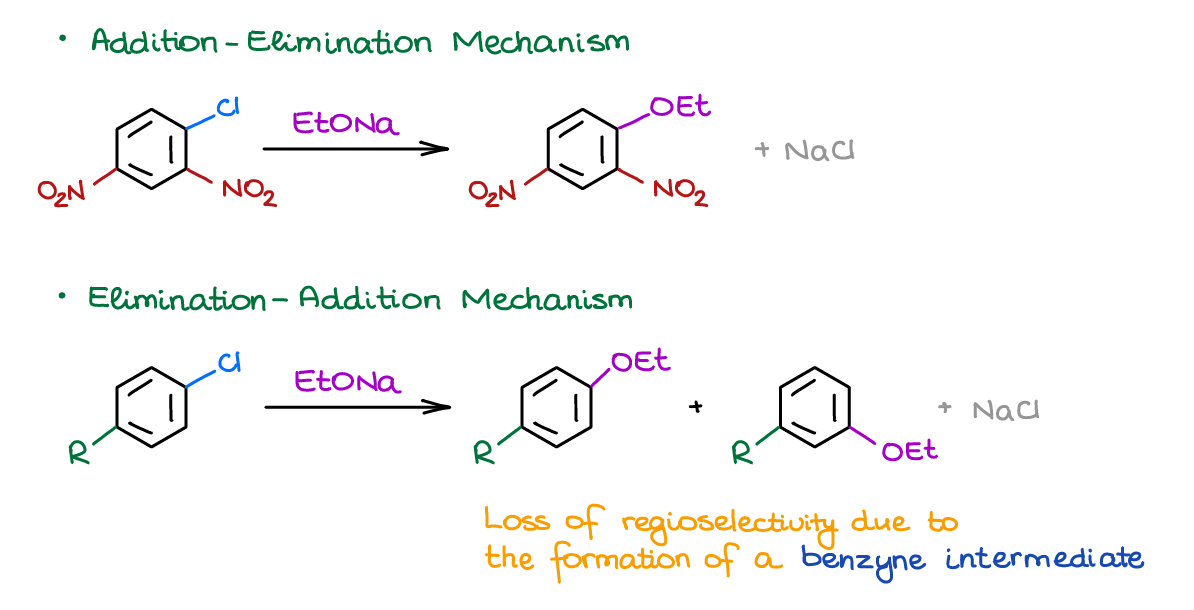
Interestingly, the nature of the substituents on the aromatic ring does matter. If you have EWG’s on the ring, you’re going to have what we call an Addition-Elimination mechanism. If you have EDG’s, you’ll end up with Elimination-Addition mechanism giving you a benzyne intermediate. Generally, you’ll always want to opt for the former rather than latter mechanism. The elimination-addition mechanism lacks the regioselectivity, so you’re going to be wasting half of your material as a result (making two isomeric products), which is not a good chemistry.
Oxidation of the Benzylic Position of the Aromatic Compounds
This is an odd reaction in which you’re going to be modifying not the ring itself but rather a substituent sitting on the ring.
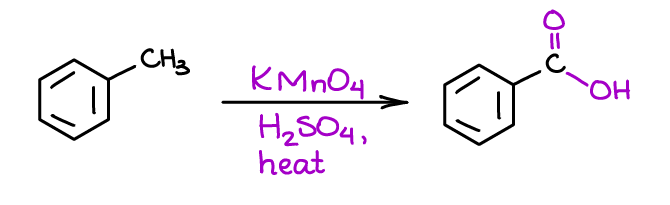
This reaction has a very marginal utility for organic synthesis as it destroys the rest of the substituent group.
Reaction of Diazonium Salts
This is a series of reactions that you may see in this unit or in the chapter on amines. This reaction is a nifty method to substitute a nitrogen-containing side group into virtually anything you like.
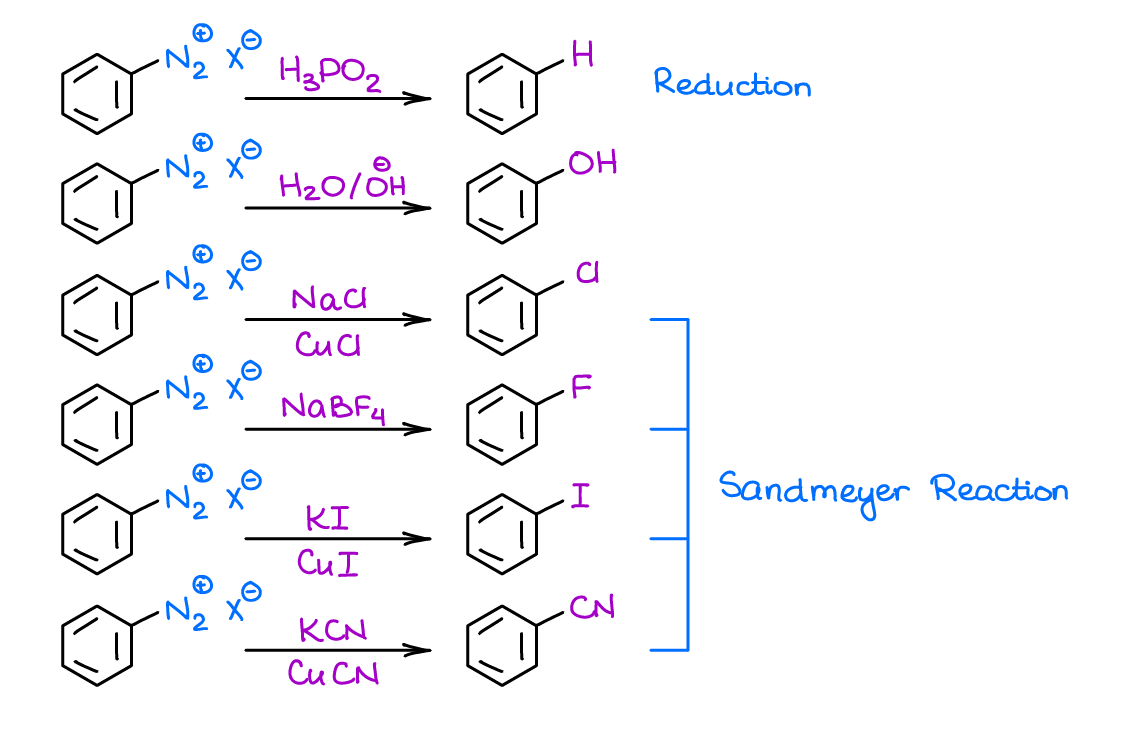
Some of those substitutions are catalyzed by Cu(I). When you use Cu(I), we call those reaction Sandmeyer reaction. Cool thing is most instructors won’t ask you for the mechanism of this fancy substitution 😀.
