NBS (N-Bromosuccinimide)
Title: N-Bromosuccinimide (NBS): A Versatile Source of Bromine in Organic Chemistry
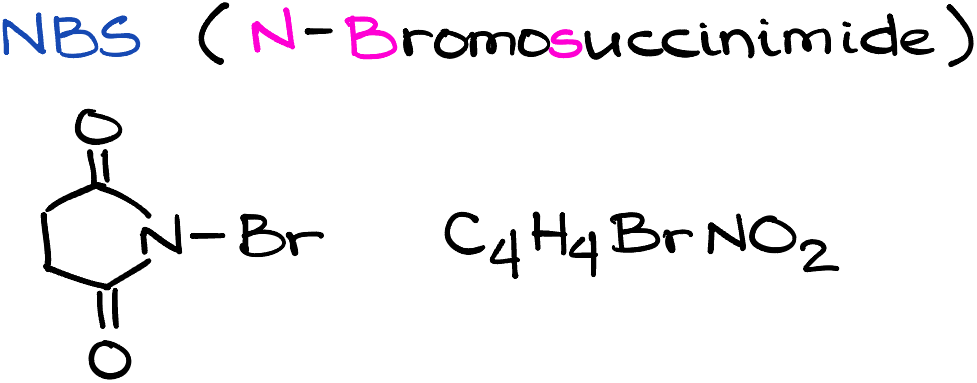
N-Bromosuccinimide, better known as NBS, is a brominating and oxidizing agent widely used in organic chemistry. Despite its humble appearance as a white crystalline solid, NBS is responsible for catalyzing several critical transformations, making it a mainstay in the toolbox of organic chemists. This entry delves into the chemical properties of NBS, its applications in organic synthesis, and safety precautions necessary when handling it.
Chemical Properties
N-Bromosuccinimide, with its molecular formula C4H4BrNO2, is a source of bromine and is frequently used when a small, controlled amount of bromine is needed. Structurally, NBS is a derivative of succinimide where one of the hydrogen atoms on the nitrogen atom is replaced by a bromine atom. This N-bromo succinimide ring gives NBS its characteristic reactivity.
NBS stands out for its capacity to facilitate selective bromination. When an allylic or benzylic hydrogen is present, NBS can selectively replace it with a bromine atom, a process known as allylic or benzylic bromination. Such specificity is invaluable when chemists want to modify a molecule without disturbing other functionalities.
Applications in Organic Chemistry
Allylic and benzylic bromination are among the most common applications of NBS in organic chemistry. These reactions can proceed via a radical mechanism under light or heat, or via an ionic mechanism in the presence of a strong acid.
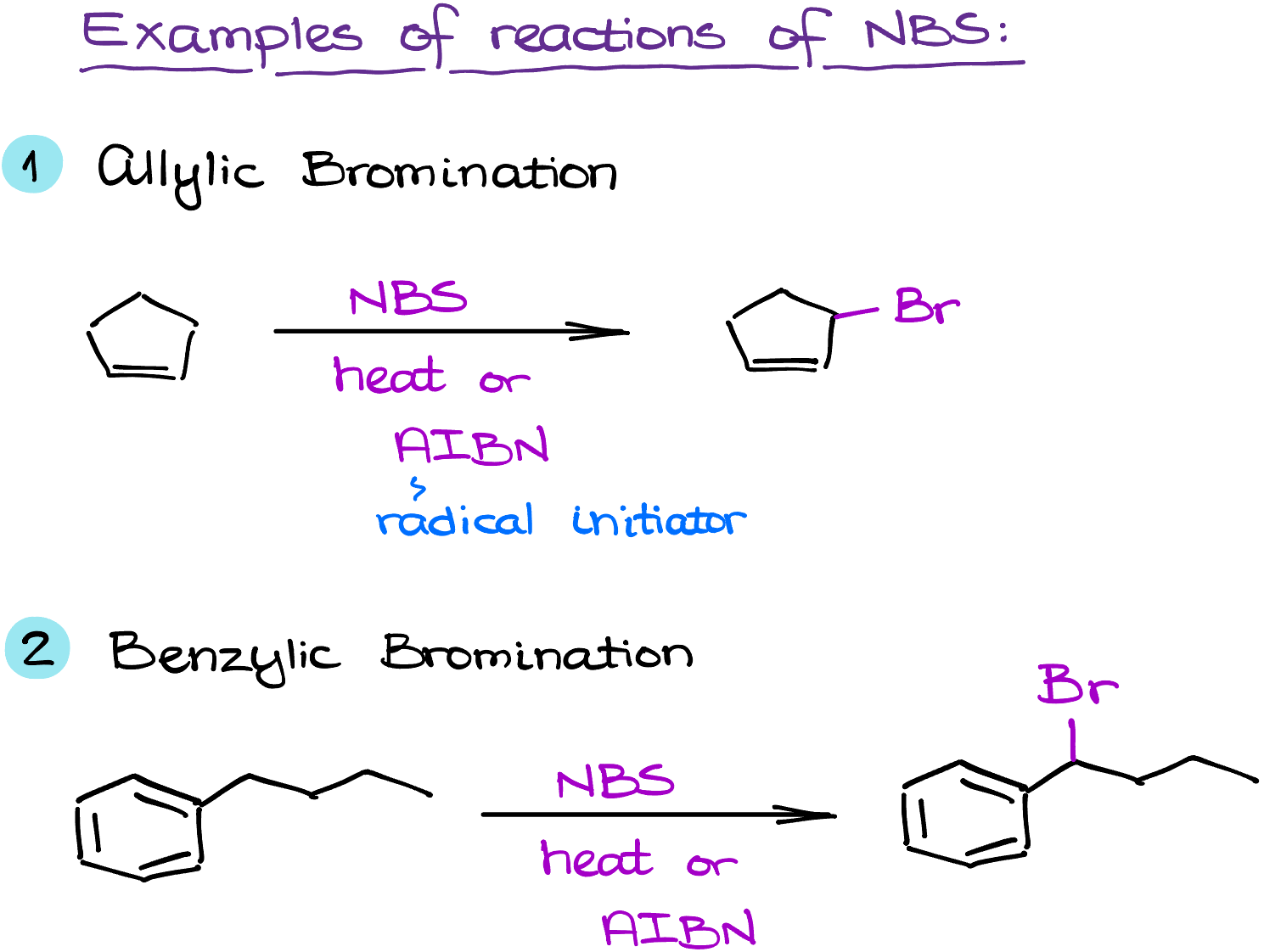
Additionally, NBS can be used in the bromohydrin formation from alkenes, the Hofmann rearrangement of amides to amines, and the oxidation of alcohols. These diverse applications underscore the versatility of NBS as a reagent.
Furthermore, the mild and selective nature of NBS makes it ideal for use in complex molecule synthesis, where conditions must be carefully controlled to ensure the desired product is obtained without affecting other sensitive functional groups.
Safety Information
While NBS is an extremely useful reagent, it is not without its hazards. NBS is harmful if swallowed, inhaled, or comes in contact with skin. It can cause skin and eye irritation, and long-term exposure may lead to more serious health effects.
Safety measures, including the use of personal protective equipment such as gloves, safety glasses, and lab coats, are necessary when handling NBS. Good laboratory practices also dictate that NBS should be used in a well-ventilated area or under a fume hood to prevent inhalation of dust.
In the event of a spill, avoid creating dust, sweep up the material carefully, and place it in a suitable container for disposal. Always dispose of NBS waste responsibly according to local regulations, as it can be harmful to the environment.
