Alcohols
Alcohol function is an extremely versatile functional group in organic chemistry. Reactions of alcohols involve oxidations, substitutions, and eliminations giving you a significant advantage in synthesis and functional group modifications. Reactions of alcohols is a typical topic in a sophomore organic chemistry and is covered in either first or second semester depending on the instructor’s choice and the textbook you’re using.
Alright, let’s get to it!
Acid-Base Properties of Alcohols
Alcohols can be a Brønsted acid or a base depending on the other components of the system. When it comes to alcohol acidity, alcohols are not considered very acidic with the pKa values ranging from 16 to 18 on average. Of course, having a strong electron-withdrawing group close to the -OH going to make alcohols more acidic. However, you’re not expected to know the pKa values outside of the normal 16-18 range. If your instructor emphasizes examples of more acidic alcohols, s/he’ll give you those on the pKa table.
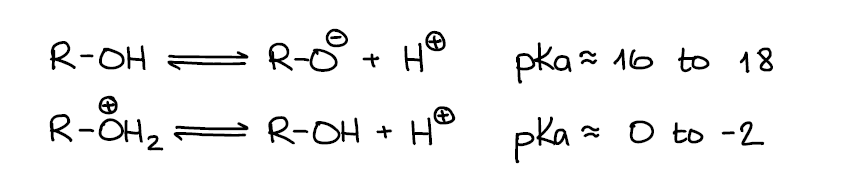
From the Brønsted base perspective, alcohols are very poor bases and can only be protonated by strong acids. Protonated alcohols themselves are very acidic with a typical pKa value close to -2. For the reference, carboxylic acids have a typical pKa around 5! Which means that a protonated alcohol is roughly 10,000,000 times more acidic 🤯.
Alkoxides as Bases and Nucleophiles
When an alcohol is deprotonated by a base, it turns into an alkoxide anion with a negative charge on the oxygen. These alkoxides are both very basic and nucleophilic, so they can participate in both substitution and elimination reactions.
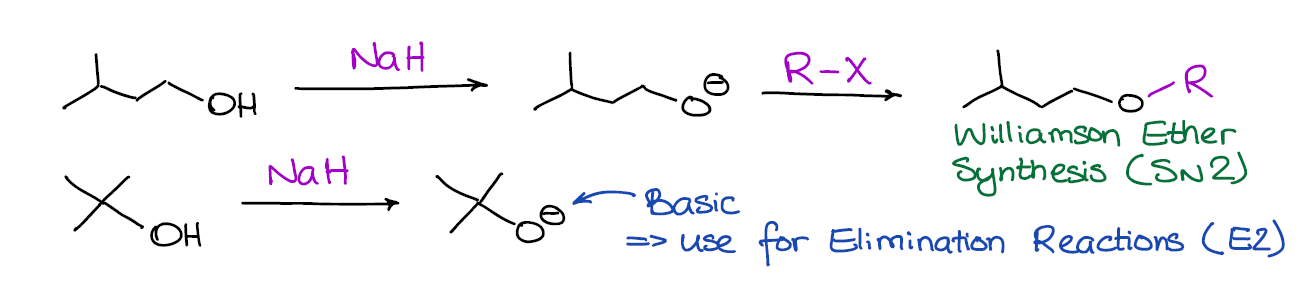
You would typically use sodium hydride (NaH) as a base in this reaction to deprotonate your alcohol for two reasons. Firstly, NaH is a very strong base (probably one of THE strongest you’re going to encounter in your course) and it has no problems deprotonating an alcohol. Secondly, the side product in this reaction is H2 gas, which makes it into a very clean reaction without any unwanted side products.
Whether the alkoxide is going to be a base or a nucleophile depends on a number of factors. If you’re covering alcohols in your class at the moment, you must have already talked about the substitution and elimination reactions. So, I would encourage you to go back and refresh your memory on the SN2 and E2 reactions. A quick reminder is that primary alkoxides are more nucleophilic while the tertiary ones are more basic.
Acid-Catalyzed Dehydration of Alcohols
This is your typical example of an E1 reaction. As the matter of fact, this is, probably, the most iconic example of E1. Pretty much every test I see out there when working with my students has acid-catalyzed dehydration of an alcohol as the E1 reaction. So, alcohol + acid is, in a nutshell, your “recipe” for E1.
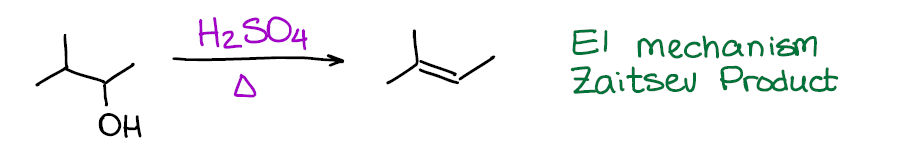
If you are thinking about a dehydration of an alcohol to make a double bond of an alkene, this is one of the worst ones out there. First, you gotta be using a strong acid like sulfuric (H2SO4) or phosphoric (H3PO4) acids that may cause other reactions. Second, this reaction has a carbocation intermediate. And, as you know, carbocations have tendency to rearrange, which only complicates things further 😭. So, when it comes to reactions of alcohols that give alkene products, there are other better options out there.
Dehydration of Alcohols with POCl3 (E2 Mechanism)
Now, this reaction is a much better dehydration option than the E1 version above. Why? It proceeds via an E2 mechanism making it easier to control. Also, no carbocation intermediate means no rearrangements. Finally, no high temperatures (unlike the E1 variant above) means no unnecessary side reactions. After all, alkenes do have tendency to polymerize when treated by strong acids, so avoiding highly acidic conditions is generally a good idea.
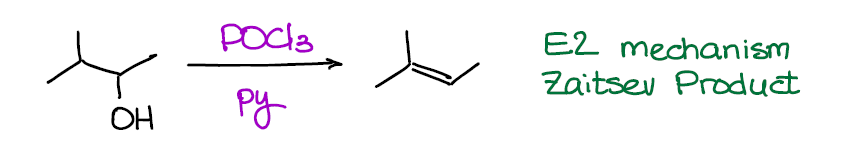
This reaction gives HCl as a side product, so we usually run it in a basic solvent like pyridine or triethylamine to neutralize it. The basic solvent is also important for the mechanism to proceed smoother, so it’s a must. Depending on how picky your instructor is about the reaction conditions, they may or may not require you to show pyridine. So, you better check in with them to make sure you’re not going to lose any point on the test for a silly omission like that.
Reactions of Alcohols with Hydrogen Halides
This is a substitution reaction what converts your alcohol into a corresponding alkyl halide. We generally use HBr for this reaction. However, there are example with HI and HCl that you may see in your course.
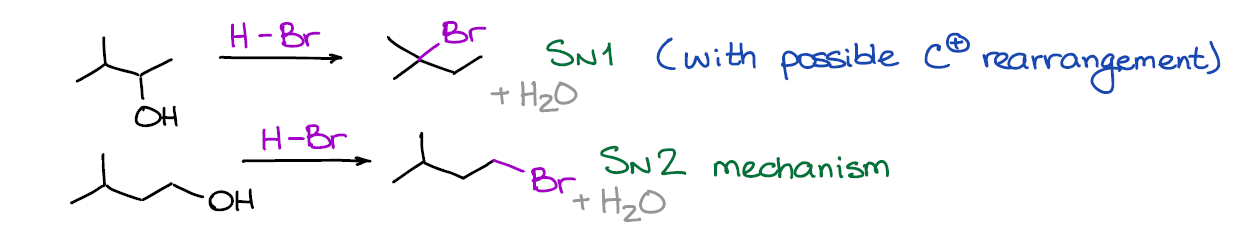
The tricky part of this reaction is the mechanism. It can be an SN1 or an SN2 reaction depending on the nature of the alcohol itself. The tertiary alcohols will always give you an SN1 mechanism, while the primary ones will give you the SN2 version. The secondary one can go either way depending on other factors and components in the mixture, but they do as well tend to generally follow the SN1 mechanism like their tertiary analogues.
Also, remember that any SN1 reaction makes a carbocation intermediate. And when you have a carbocation, you may have rearrangements, so always check for those.
Alcohol “Activation” via Sulfonyl Esters
Since the -OH is a pretty bad leaving group (and a very strong nucleophile too), you’re unlikely to get a direct substitution reaction of the -OH without first modifying it. In the case of dehydration or reactions of alcohols with HX, the “modification” happens in situ via the protonation of your -OH group and turning it into a decent leaving group (H2O). Sometimes, however, doing a reaction in acidic conditions is not such a good idea. So, what can you do in such a case? Well, we’re going to convert our alcohol into a good leaving group by turning it into a sulfonyl ester!
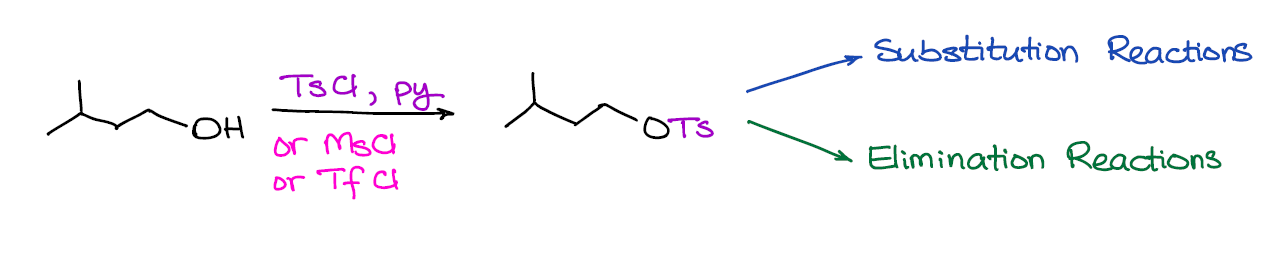
What’s really cool about this reaction is that it opens a lot of doors for your synthesis and you’re not limited by what you have in your reaction mixture at the moment. As sulfonyl esters are easily isoluble from the solution, you can make them and keep them for the future use.
An important thing to remember is that sulfonyl esters is a family of compounds. You’ll probably see tosylate, triflate, and mesylate in your course. If your instructor is creative, they might throw in a few more into the mix. What I’ve seen instructors do is to give you actual structures instead of abbreviations. So, make sure you do know the structures of your reagents! Otherwise, you’re running a risk of mistaking your reagent with something else and do the wrong thing on the exam.
Conversion of Alcohols into Chlorides
If you want to run your conversion of alcohols into a chloride in a predictable way, you want to avoid carbocations by all means. One of the convenient ways to do that is by using one of the following reagents: SOCl2, PCl3, or PCl5. These reactions usually run in pyridine as a solvent, although they can be done neat (without a solvent).
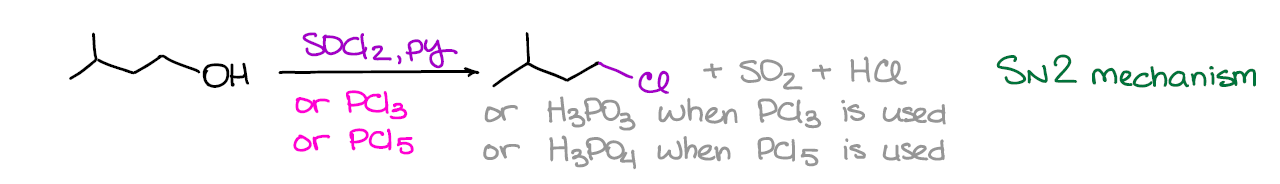
This reaction generally follows the SN2 mechanism, however, some exceptions are known. Also, since it does indeed prefer SN2, it works the best for primary alcohols.
Conversion of Alcohols into Bromides
Just like in the example above, you can just as easily convert alcohols into bromides using similar reagents.
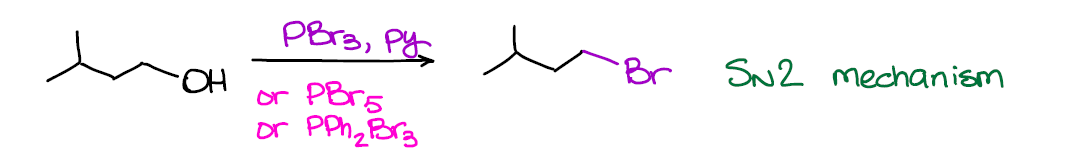
And also like in the previous example, this reaction is SN2 and works best for primary alcohols. Conversion of alcohols into bromides is generally a milder reaction and gives better yields. So, it’s the one that you’re going to be using the most in your course.
Oxidative Cleavage of Vicinal Diols
When you have two alcohols on the adjacent carbons you can break the C-C bond between them using periodic acid. This reaction gives the same result as the reductive ozonolysis of alkenes and can be used as an alternative when the ozonolysis is not an option.
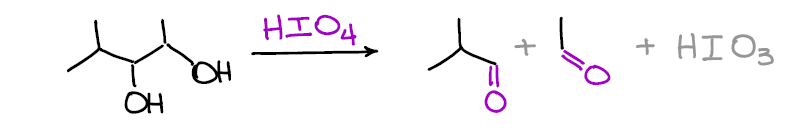
What’s cool about this reaction is that it’s very selective. This means that other functional groups you might have in your molecule are safe!
Oxidation of Alcohols
This is a very important topic in this chapter! There are many ins and outs in here so you definitely want to pay extra attention when reading your textbook and listening in the lectures.
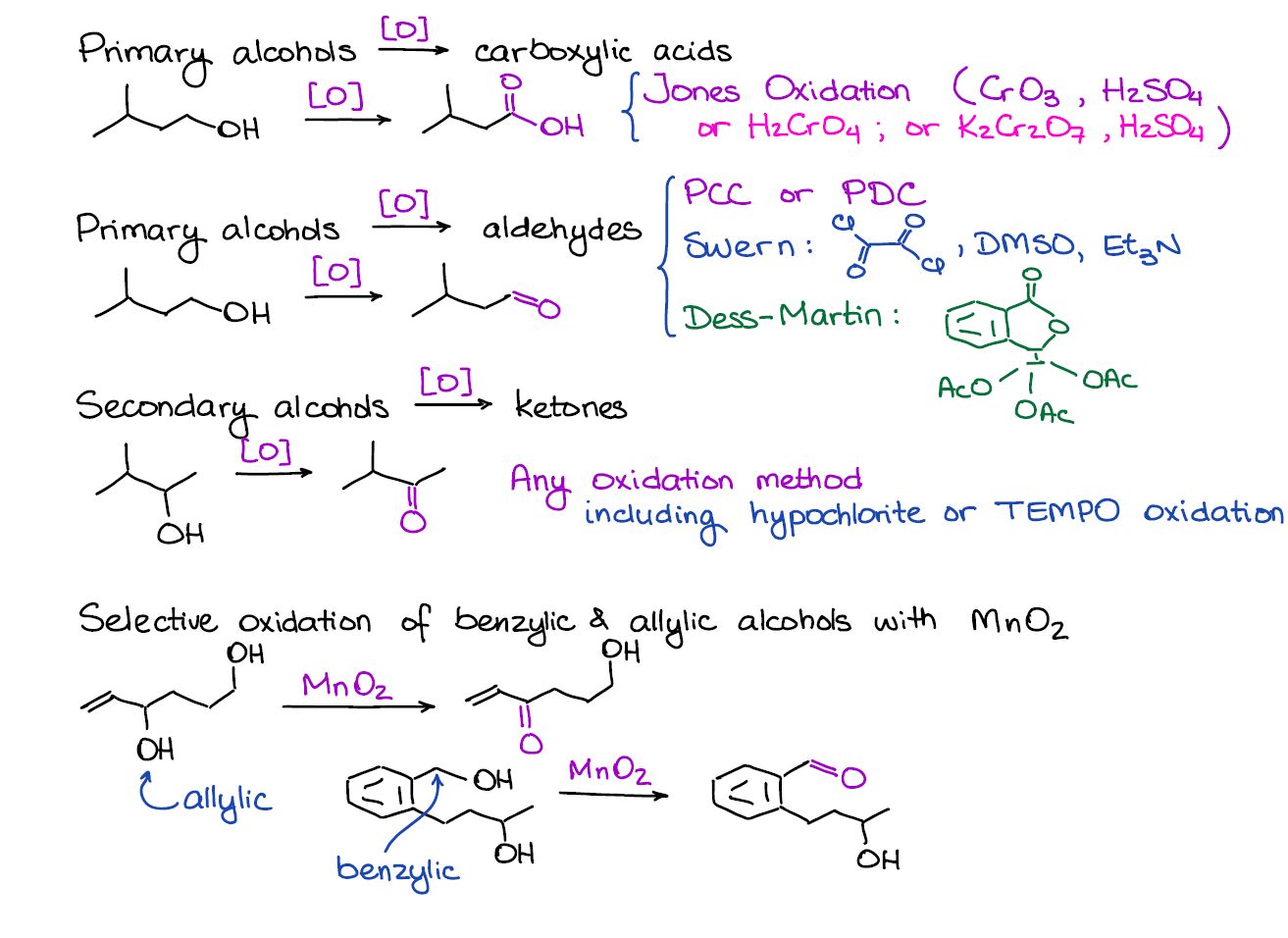
First, it’s important to recognize if you have a primary or secondary alcohol that you’re trying to oxidize. Primary alcohols can go through two oxidation stages giving you two different products depending on the oxidizing agent. Moreover, if you have benzylic or allylic alcohols, those have a very selective oxidation reaction that only works for them and not other alcohols.
This block will also have a bunch of mechanisms. I often see instructors skip the mechanisms for the alcohol oxidations as they tend to be a little long and tedious. However, some love putting those on exams 😖. So, keep your ears sharp and listen carefully if that’s something you need to know for the exam or not.
Did you like this introduction to the reactions of alcohols? I have a whole set of notes on this topic with the detailed description of all reaction with ALL mechanisms that you wanna know to ace your test!

thank you
You’re very welcome!
“Dehydration of Alcohols with POCl3 (E2 Mechanism)
Now, this reaction is a much better dehydration option than the E1 version above. Why? It proceeds via an SN2 mechanism making it easier to control.”
In the second sentence of this paragraph it says that dehydration of alcohols proceeds via SN2. Is this a typo or have I missed something 🙁 ??
Oh! It certainly is a typo, it should be E2, of course. I’ll fix it. Thank you for noting it!