Amines
In this lesson we are going to go over the most typical ways of amine synthesis and reactions of amines. While many curricula skip this topic or merely brush upon it, organic chemistry of nitrogen is extremely important. It is especially important if you want to understand many biochemical processes and pathways.
Synthesis of Amines
There are many ways how you can make an amine. Here, I want to go over the most common ones going from roughly the least useful to the most useful.
Direct Substitution of Alkyl Halides
The direct substitution of alkyl halides is a simple SN2 reaction. In this reaction you will react primary or secondary alkyl halide with ammonia or an amine.
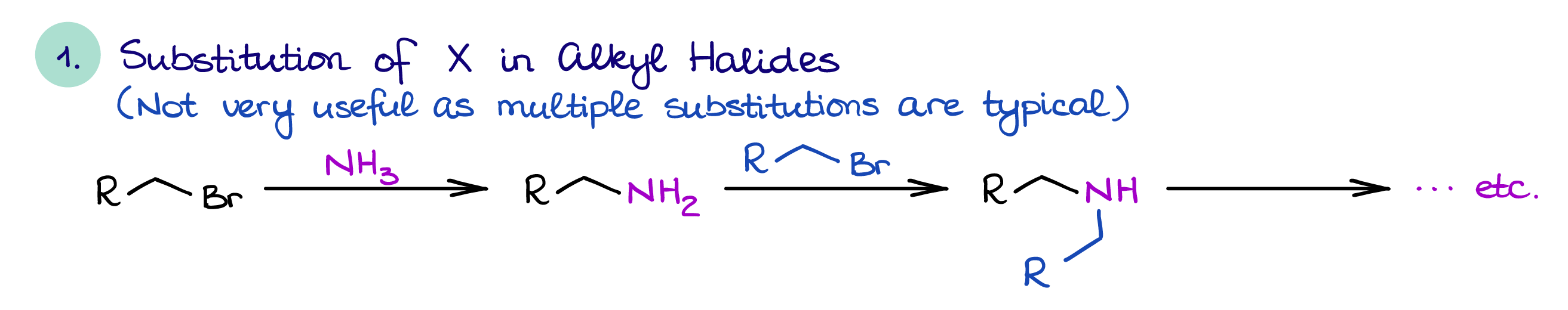
This way of amine synthesis is not very useful because the resulting amine is typically more nucleophilic than the original nitrogen-containing species making it more reactive. This usually leads to overalkylation and a mixture of products. Because of that, this reaction is only feasible on an industrial scale where we would typically use huge excess of ammonia to minimize the extent of an overalkylation.
Reduction of Nitrogen-Containing Functional Groups
The nitrogen-containing functional groups that you would normally reduce to make an amine are:
- Amides
- Nitriles
- Azides
And while other functional groups sometimes pop up in textbooks and quizzes, these are the most common ones.
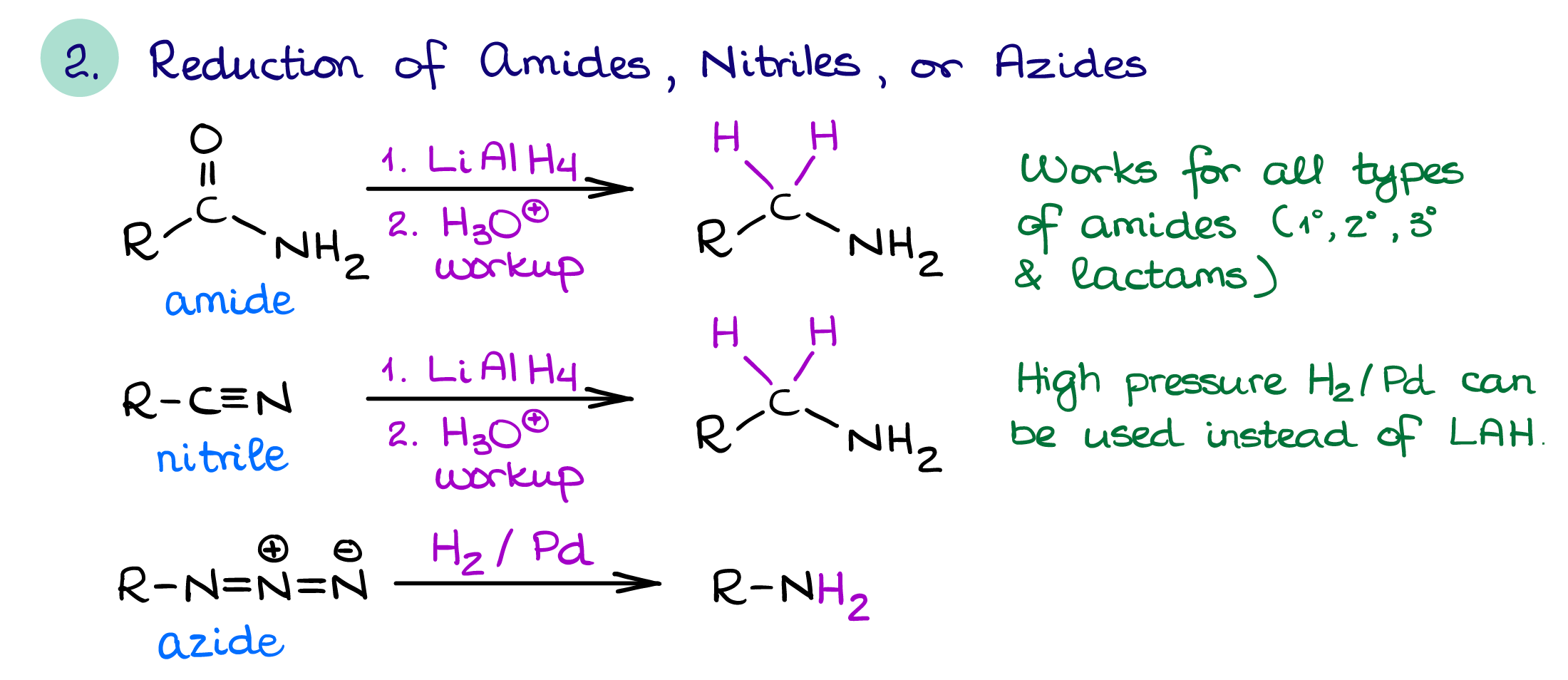
Nitriles and azides can be easily prepared via a simple SN2 reaction. This makes the reduction of nitriles and azides with complex hydrides like LiAlH4 or hydrogen on heterogeneous catalyst (Pt or Pd) are the most useful examples of this method of amine synthesis. Remember though, the reduction of a nitrile which was prepared via an SN2 reaction will add an extra carbon to the chain. Reduction of cyclic amides (lactams) is especially useful as you can make nitrogen-containing heterocycles this way which may be difficult to synthesize otherwise.
Hofmann Rearrangement
The Hofmann rearrangement is a useful way of amine synthesis that also allows you to shorten the chain by one carbon (yes, sometimes it’s useful 😉). As I’ve mentioned above, amides can be simply reduced to make a corresponding amine. The reduction of amides preserves the length of a carbon chain. Also, you can reduce either 1°, 2°, or 3° amides. The Hofmann rearrangement only works for the 1° amides.
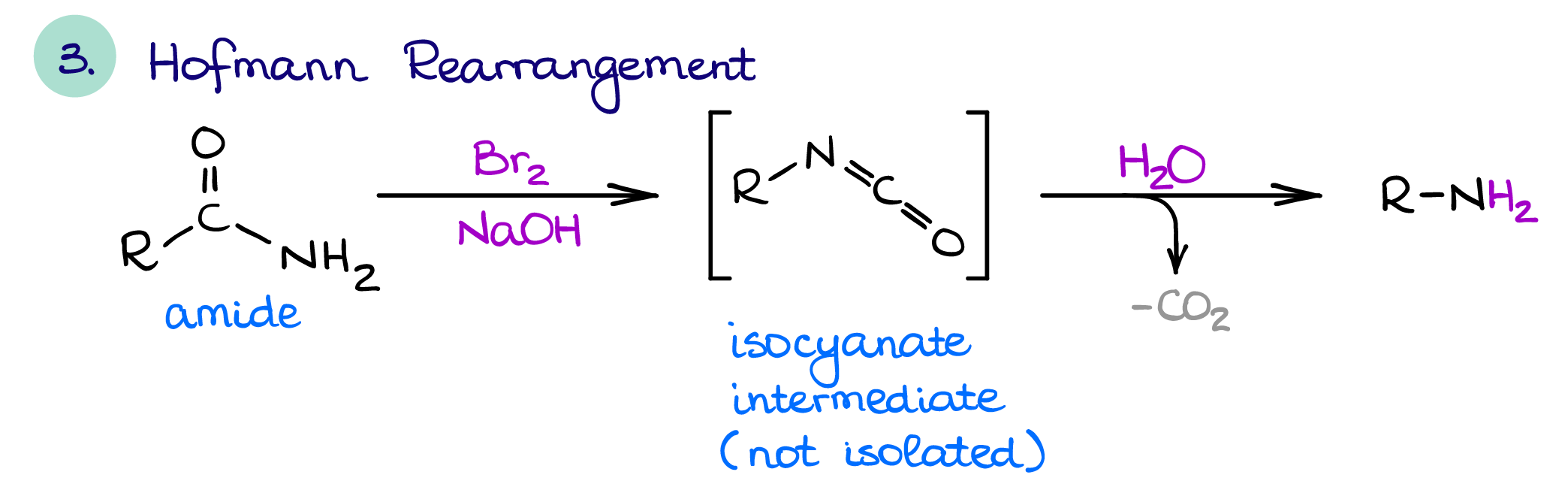
This reaction has a rather interesting mechanism, which makes it a perfect candidate for a test question. So, if you cover it in class, make sure you know the mechanism so you can ace that test.
Curtius Rearrangement
The Curtius rearrangement is pronounced [Koor-tsee-oos] after the German professor of chemistry Theodor Curtius and it has nothing to do with “courtesy” 🤣. Anyways, the Curtius rearrangement is quite similar to the Hofmann rearrangement.
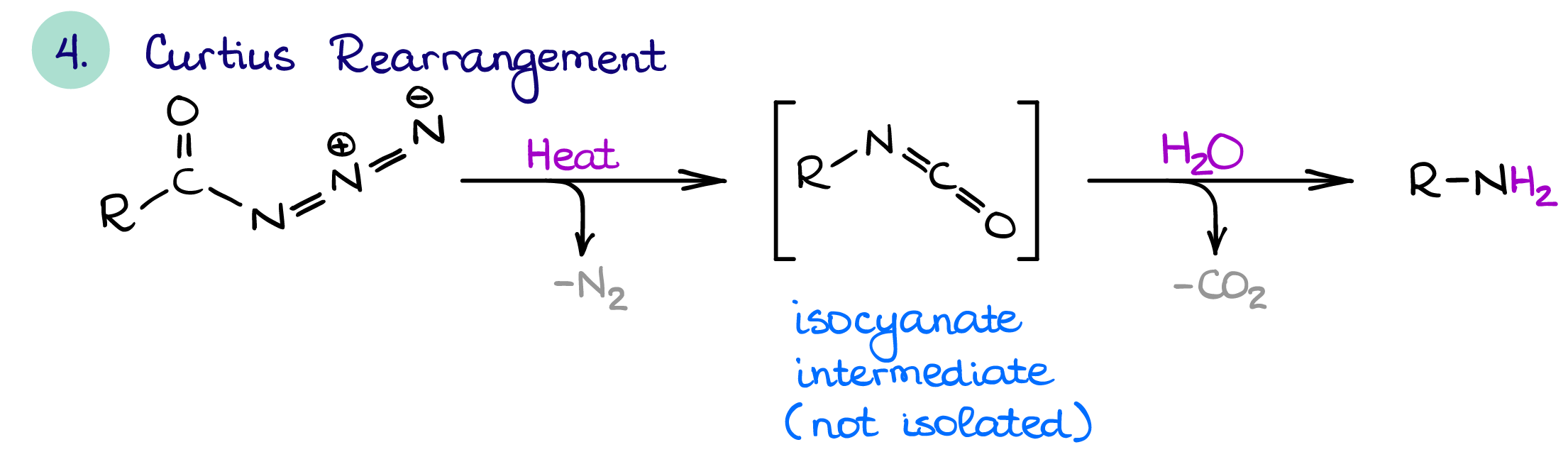
Both Curtius and Hofmann rearrangements have similar mechanisms, both result it one carbon chain shortening, and both have an isocyanate intermediate. The only difference is the starting material.
Reductive Amination
The reductive amination is the most useful amine synthesis strategy. It affords functionalized amines and doesn’t typically give many unwanted side products. It can also be done single pot making this reaction very useful for the modern synthesis as it allows us to cut the time requirements.
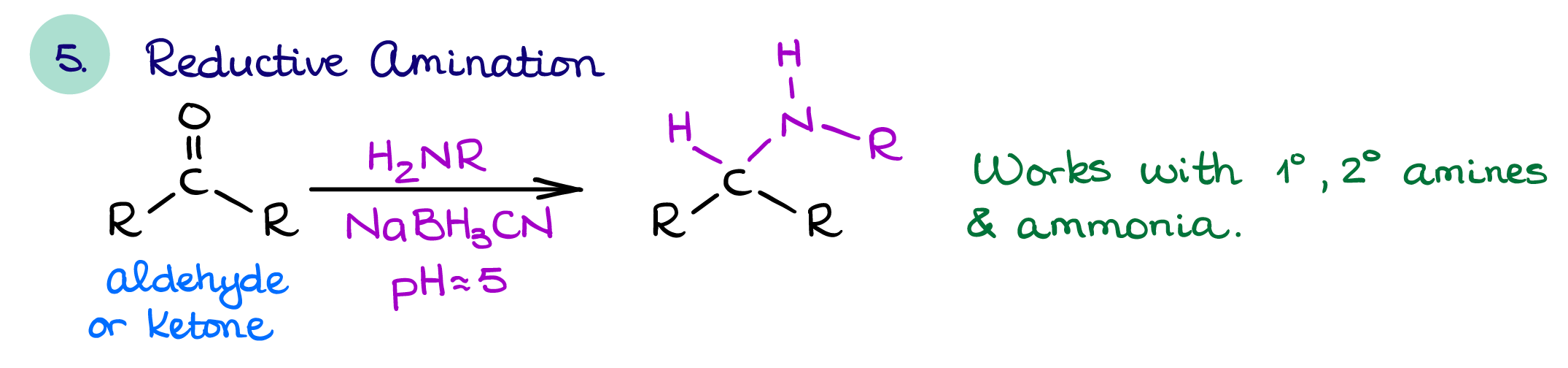
If you’re planning an amine synthesis, you should always try using this reaction. Especially if you’re trying to make some sort of highly functionalized amine with complex substituents on it. This reaction is very versatile and can yield a vast range of amine products.
Reactions of Amines
Amines are good nucleophiles and are also weakly basic. This means that we always need to pay close attention to the possible acid-base reactions in their presence along with possible substitutions (SN2) or even occasional eliminations (E2).
Acid-Base Properties of Amines
Often, you’ll see amines as bases as they can be easily protonated by acids.
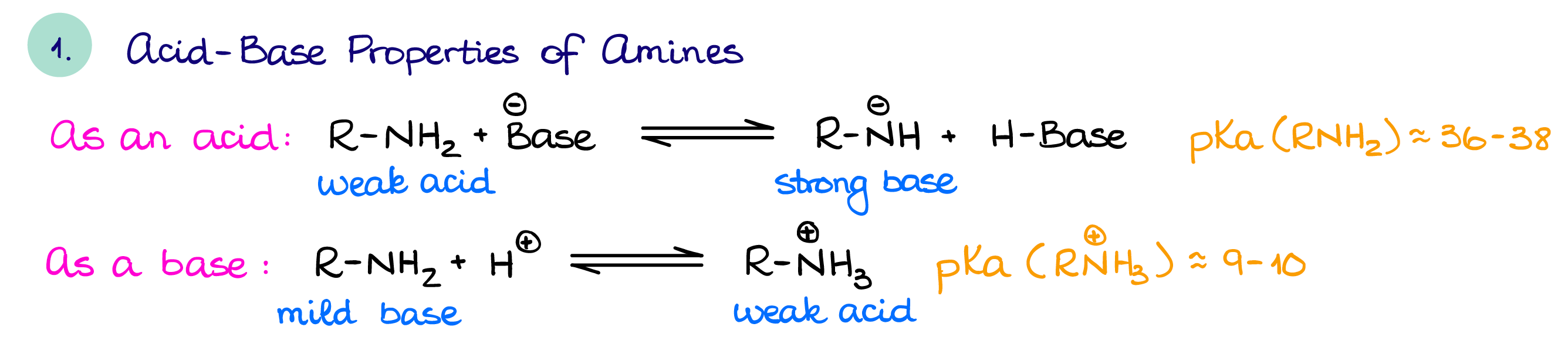
A protonated amine is, in turn, slightly acidic. If we, however, deprotonate an amine, we’ll make an exceptionally powerful base. The most common super strong nitrogen-containing base is LDA (lithium diisopropylamine) which you would typically use when you need a very strong and a very bulky base. Other nitrogen-containing bases such as DBN/DBU, triethylamine, and pyridine are quite common in reactions requiring bases or basic conditions.
Amines as Nucleophiles
Amines are also good nucleophiles which makes them good reagents for the substitution reactions (mainly, SN2).
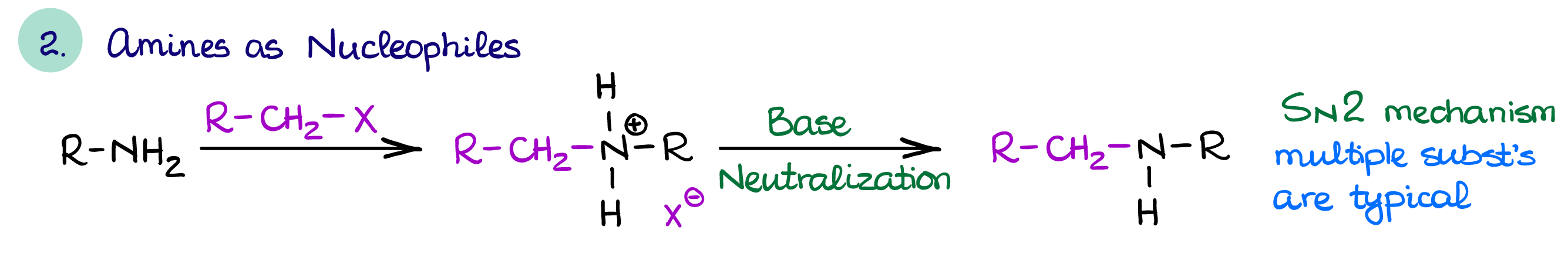
Using amines as nucleophiles is, however, something that we often avoid dues to the overalkylation problem. As I’ve already mentioned earlier on this page, amines with more alkyl groups are typically more nucleophilic than those with less substituents making the reaction products more reactive than the reactants. So instead, we’ll try to use the reductive amination to functionalize our amine when possible.
Diazotation of Amines
This is a rather interesting and unique reaction of amines that gives different results depending on a presence of a hydrogen in the α-position next to the nitrogen atom.
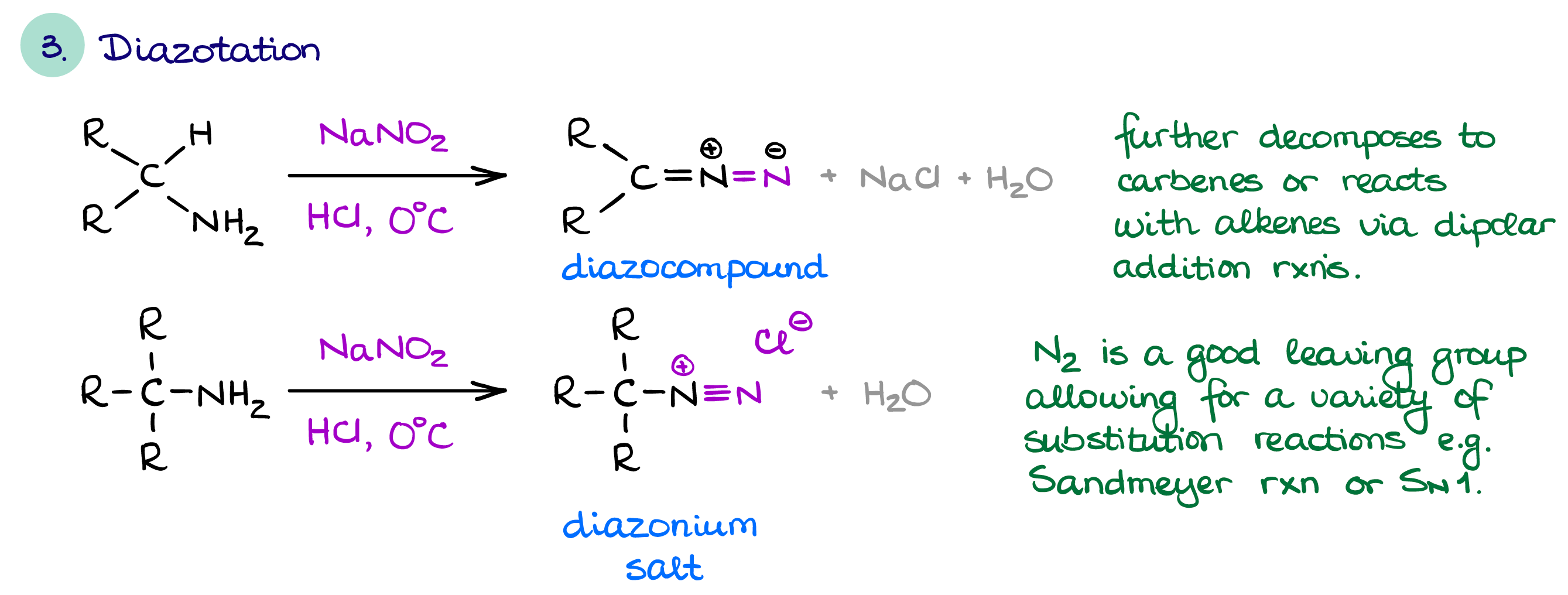
If there’s an available hydrogen next to the nitrogen atom, we are going to make a diazocompound. Diazocompounds have a wide range of unique chemistry associated with them. They can do dipolar cycloadditions, insertion reactions, and carbene chemistry.
If, however, a hydrogen is not available, then you’ll make a diazonium salt. The best part of the diazonium salt is that N2 is the best leaving group in existence. Thus, the diazonium salts undergo various substitution reactions with ease. Additionally, the N2 group in the diazonium salt is electrophilic and can act as an electrophile in an electrophilic aromatic substitution. EAS with a diazonium salt gives the -N=N- azo group which is an industrially important chromophore. The chemistry of diazocompounds and diazonium salts is very diverse for a few short paragraphs here, so I’ll put it in a dedicated section.
Hofmann and Cope Eliminations
The two unique reactions of amines are the Hofmann elimination and the Cope elimination.
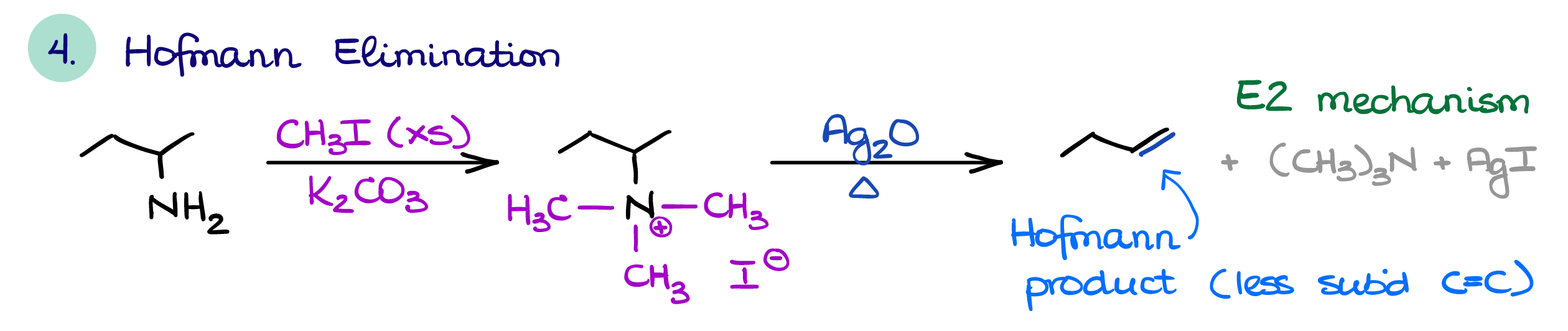
In the Hofmann elimination, we first perform an overalkylation of an amine. We then use resulting quaternary ammonium salt as a leaving group. An important feature of the Hofmann elimination is that it always gives you the least substituted product. This is somewhat counterintuitive, as we typically want a more substituted alkene. But the nature of the transition state required for this E2 reaction dictates the formation of the least substituted alkene as the major product. Other than that, it follows all the rules and requirements for the E2 reaction.
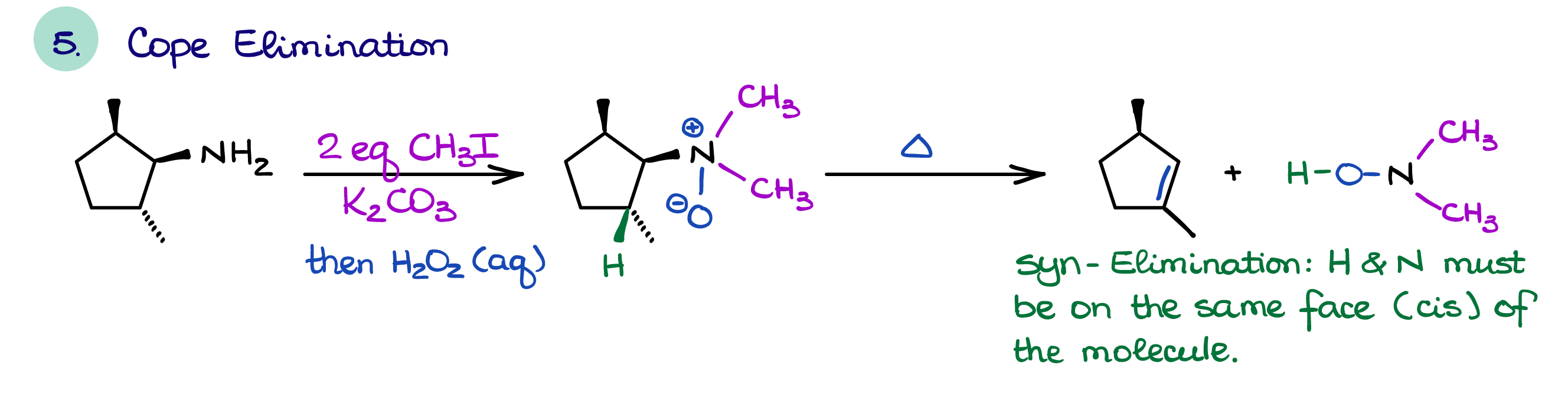
The Cope elimination is similar to the Hofmann elimination as it requires the formation of an alkylated amine. However, unlike the Hofmann elimination, in the case of the Cope elimination we make a base a part of the leaving group itself. This forces reaction to follow the syn-elimination mechanism. Thus, it has a completely opposite transition state than a typical E2 reaction which requires the leaving group and the proton to be anti to each other.
