Acid-Base Chemistry
Acid-Base chemistry is a big topic in organic chemistry. Most reactions you will look at within the scope of the course will have some acid-base aspects to them. So, it’s essential that you pay close attention to this topic.
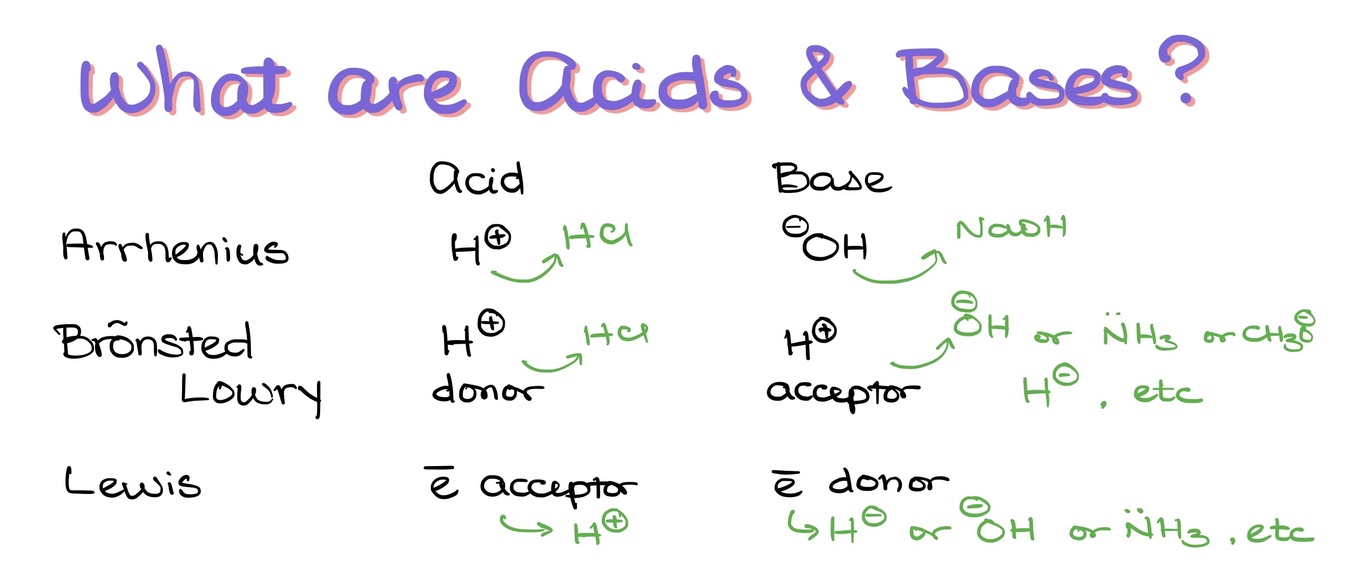
There are three major theories that we see in a typical general and organic chemistry courses. Those are the Arrhenius theory, the Bronsted-Lowry theory, and the Lewis theory.
Arrhenius Theory
Arrhenius theory is only applicable to the aqueous solutions. In AT, the acid is the donor of H+, while the base is the donor of the OH–. As in organic chemistry we rarely deal with aqueous solutions, the Arrhenius theory is virtually never used.
Bronsted-Lowry Theory
BL theory of acids and bases (AB) is a more useful theory within the scope of organic chemistry as it focuses on the proton (H+) transfer. BLT classifies an acid as a species that donates a proton into a solution, while the base accepts it. As this process can happen in any type of media, the BL theory is perfectly applicable to organic systems.
The Bronsted-Lowry theory (BLT) describes acid-base (AB) equilibrium as a transfer of a proton (H+) from one species to another.
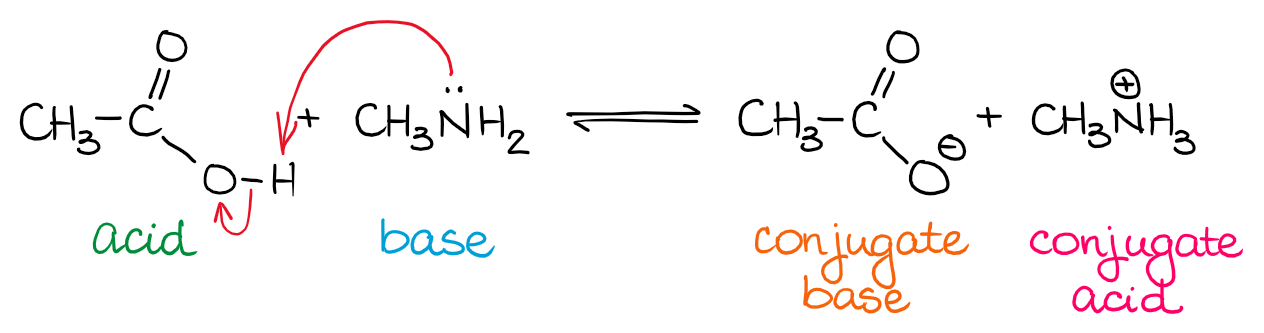
In a typical BL AB reactions, the source of the proton (H+) is an acid and the H+ acceptor is a base. Once the acid loses the H+, it becomes a conjugate base. Likewise, once the base accepts the H+, it becomes a conjugate acid. The term “conjugate” refers to the product of the reaction in the acid-base equilibrium.
How to Identify an Acid and a Base
You can easily identify a BL acid or a base by carefully following the movement of the proton from one species to another. Since the proton donor is an acid, whatever species loses it is an acid. Likewise, whatever gets the H+, is the base!
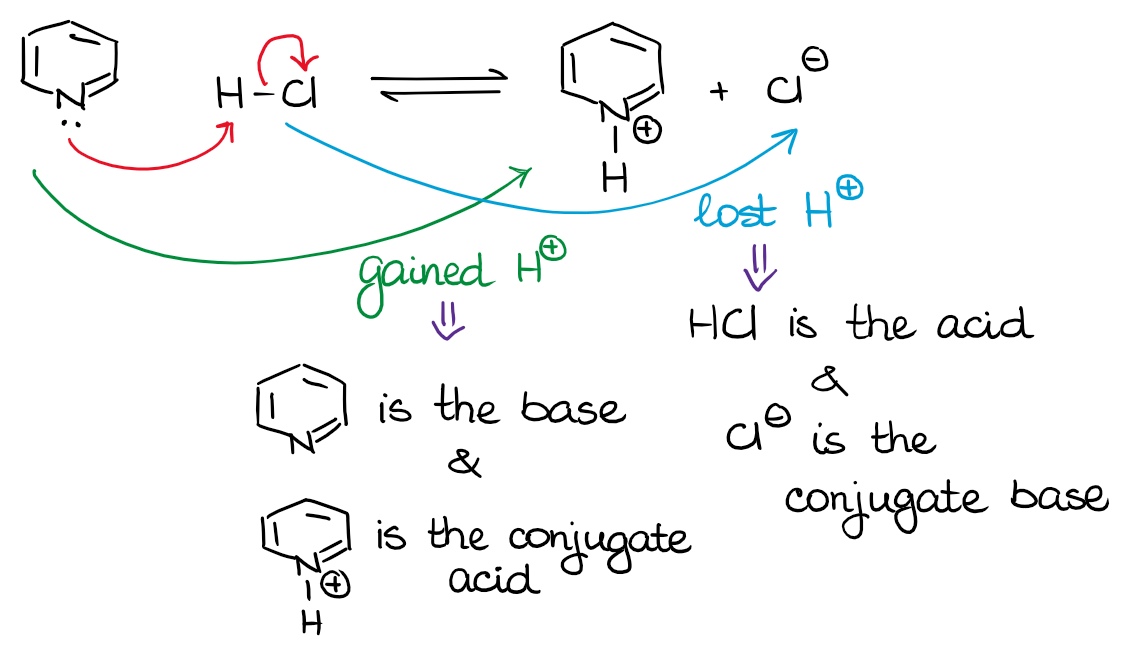
In the example above, the pyridine (a cyclic compound with the nitrogen) is a base. It accepts the proton from HCl and becomes the conjugate acid on the other side of the equilibrium. And, since the HCl donated the H+ into the system and gave it to our pyridine, it is our acid. Once it loses the proton, it becomes the conjugate base.
What is the Difference Between a Strong and a Weak Acid?
Strong acids dissociate completely. Thus, when we say that something is a strong acid, it means that it all has dissociated by donating its protons to the solution. HCl is an example of a strong acid. In a solution there will be virtually no undissociated HCl.
Most organic acids are, however, weak acids. We characterize weak acids with their dissociation equilibrium constants (Ka). For instance, let’s look at the dissociation of a typical organic acid, acetic acid:
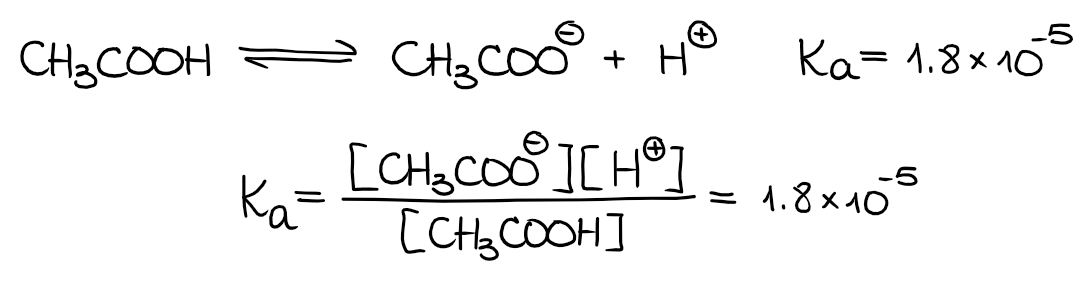
By definition, the Ka is a product of the concentrations of the products of the acid dissociation over the concentrations of the leftover acid. For the acetic acid, the dissociation constant Ka = 1.8·10-5. This is a very small number, which means only a very small portion of acetic acid actually dissociates in the solution and the majority of CH3COOH stays as is.
The Ka values are quite inconvenient for a everyday use. Instead of the Ka values, we typically use the pKa values. By definition, the pKa = -logKa, thus:
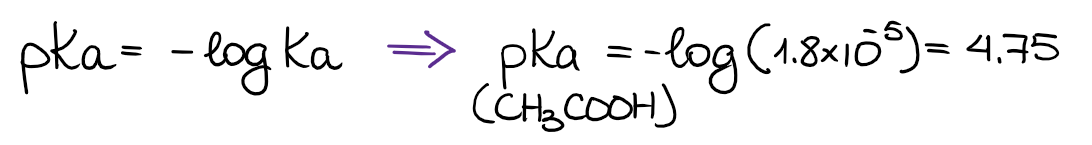
Typical pKa values of organic substances are usually somewhere between 2 and 18. Of course, there are compounds with drastically larger or lower pKa values. But those are going to be some special molecules that we’ll be using as very strong bases or acids in the future.
For now, remember:
Small Ka = weak acid
Large Ka = Strong acid
Small pKa = strong acid
Large pKa = weak acid
As the Ka and pKa have an inverse relationship, it’s very important to be very careful when reading the question! Your instructor can easily trick you by asking you to arrange molecules according to their pKa’s, Ka’s, or acid strengths. And if you’re not paying attention, you can give an opposite ranking for the answer.
Lewis Theory
LT adds an extra layer onto the AB discussion. In the LT, the acid is the acceptor of the electrons, while the base is the donor of electrons. A notable thing about a typical LT AB equilibrium is the formation of a new donor-acceptor bond between the acid and the base. We use the LT when we’re dealing with the equilibria that do not involve a proton transfer.
Lewis theory sees the acid-base equilibrium as the movement of the electron pair from one compound to another. The hallmark of the Lewis acid-base equilibria is making of a new donor-acceptor bond between an element that was a donor of electrons and an elements that accepts those electrons.
Thus, the acid in the Lewis theory is defined as an acceptor of the electrons. The base is a donor of electrons. It’s very important to remember this difference between the Lewis theory and the Bronsted-Lowry! Otherwise, it’s really easy to get all confused on the test! 😉
So, let’s look at a couple of examples of the Lewis acid-base equilibria:
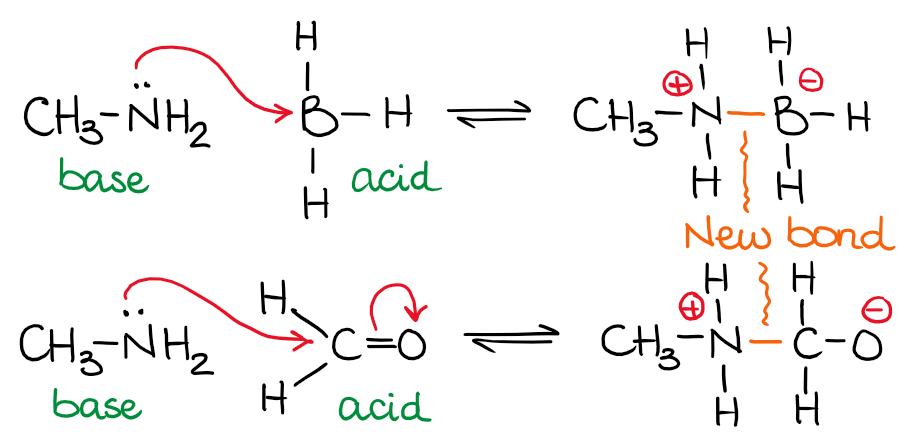
In the examples above, I have the same base but two different acids. The amine (nitrogen-containing base) donates its spare electron pair on the nitrogen to make a new bond with boron or carbon. Notice, that donating electrons to another atom will decrease the electron density on the donor making it neutral if it was negatively charged or positively charged if it was neutral. Likewise, an acceptor of electron density will change its charge from neutral to negative or from positive to neutral.
Generally, we’re not going to classify the Lewis acids as strong or weak as it’s quite difficult to do. So, for the most part, we’re going to assume that all Lewis acids we work with are strong enough for our purposes. We’ll also see an very limited number of those, so you don’t really need to be concerned with their strength. The main focus will be always to identify one.
