Enolization of Carbonyls
In the previous tutorial, we’ve talked about the factors that affect the acidity of carbonyls. In this tutorial, I wanna talk about the effects of the base on the enolization of carbonyls equilibrium. Let’s look at a simple equilibrium between a ketone and some sort of a base.
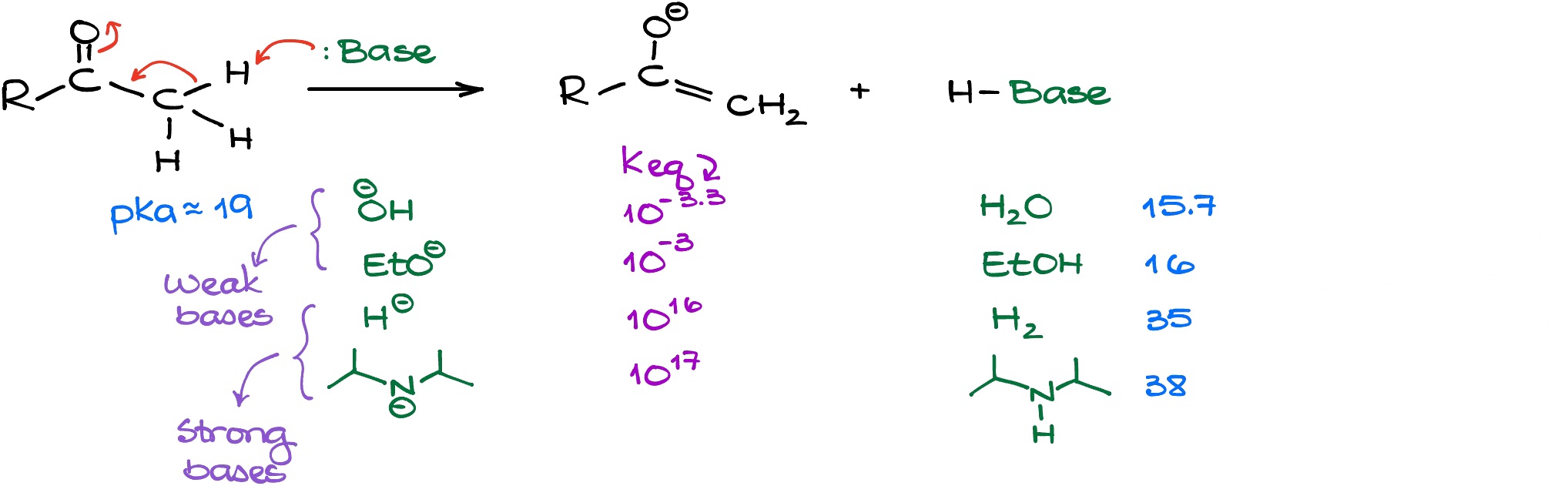
Since this is fairly common acid-base equilibrium, we can easily estimate the equilibrium constant for this reaction if we know the nature of the base that we use in this reaction. In the table above, I have outlined a few common bases that we see in organic chemistry. Looking at the equilibrium constants (Keq) we have for each of those equilibria, we can see that we can separate the bases into two categories: strong bases and weak bases.
The weak bases (hydroxide and alkoxides) will only give a small quantity of the enolate. The strong bases (hydrides, LDA, etc.), however, will enolize our ketone completely.
Enolization of Dicarbonyls
Remember how I emphasized that you need to know your pKa values in the Acidity of Carbonyls tutorial? Well, I always emphasize that for a reason.
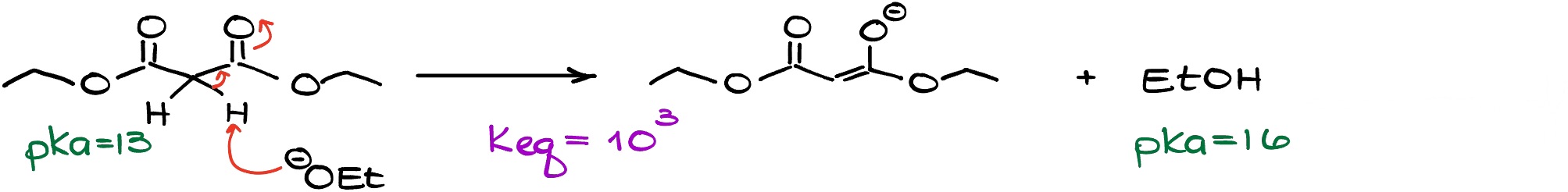
If we look at the reaction above, the starting material is acidic enough so even a weaker base will be able to deprotonate it completely. So, if we wanted to make a solution that is 100% enolate, we no longer need to use a powerful base and work with something milder.
Competitive Enolization: Thermodynamic vs Kinetic Enolates
It’s easy to predict the structure of the enolate when we only have one enolizable ⍺-position. However, things become a bit more complicated when we have multiple enolizable positions. For instance, let’s look at the following reaction:
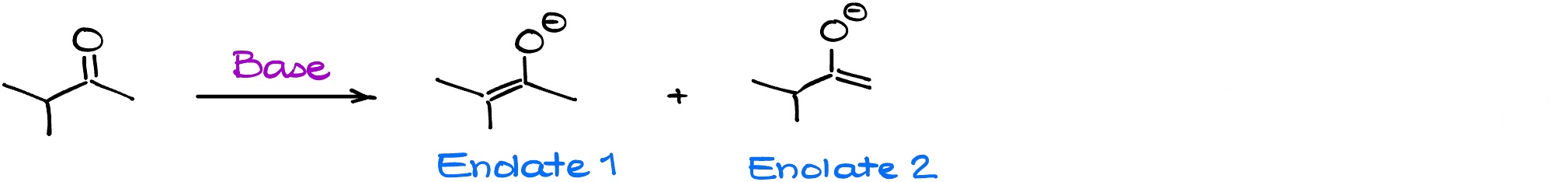
We have two possible enolates as our potential product. Are we going to have them both present in the mixture? Yes! Are they going to be present in similar quantity? Depends…
If we’re using a weaker base like hydroxide or alkoxide, this reaction will have a rather small equilibrium constant meaning that we’re going to be constantly at equilibrium with the starting material (ketone).
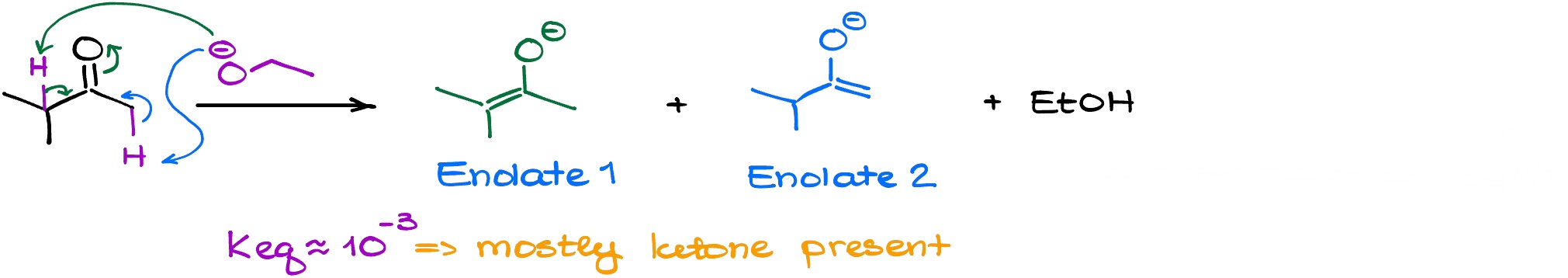
And since we’re at an equilibrium, the more stable product is going to be present at a higher concentration. But which enolate is more stable?
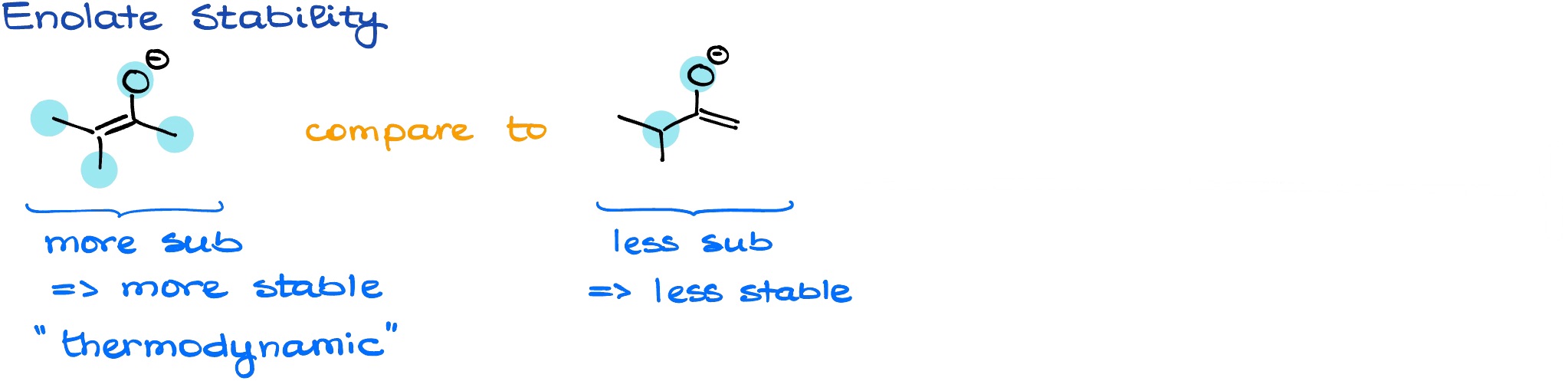
Typically, we’re going to pay attention to the number of substituents around the double bond on our major resonance contributor. The more substituted the double bond is, the more stable that enolate is going to be. We call such enolate a thermodynamic enolate. There are, or course, exceptions from this general trend, but for the most part, it’s going to be out guiding rule of thumb.
Using Strong Bases to Make Thermodynamic Enolates
Now, the reaction above only produces a small quantity of the enolate. Is there a way we can push this equilibrium towards the enolate more substantially? Absolutely! We need to satisfy two conditions:
- We need a strong base,
- We need to perform the reaction in such a way as to allow for the system to constantly reach the state of equilibrium.
Typically, we’re going to use one the following two scenarios.
In the first scenario, we’re going to use a strong slow-acting base, such as NaH (sodium hydride). Because sodium hydride is an ionic compound and doesn’t dissolve an anything that even remotely resembles an organic solvent, it’s always used as a suspension. And since the molecules will be reacting on the surface of the granules of the NaH solid, we’re always going to have the excess of the starting material (ketone) in the solution allowing for the equilibrium between the enolates and the ketone.
In the second scenario, we’re going to use the other powerful base that we typically see in organic chemistry – lithium diisopropylamine (LDA). BUT! Quantities and addition order matters here! As I’ve mentioned above, we need to run our reaction in such conditions where we allow for the equilibrium. This means that we should always have some of your ketone present in the system. So, if you slowly add LDA (which is soluble in many organic solvents) to the solution of your ketone, you’ll always have surplus of the ketone.
This might not be an obvious trick, so let me explain it in more details. LDA is known as a kinetic base. This means that once LDA “sees” a proton, it will snatch the “easiest” proton to grab, which is the least sterically hindered one.
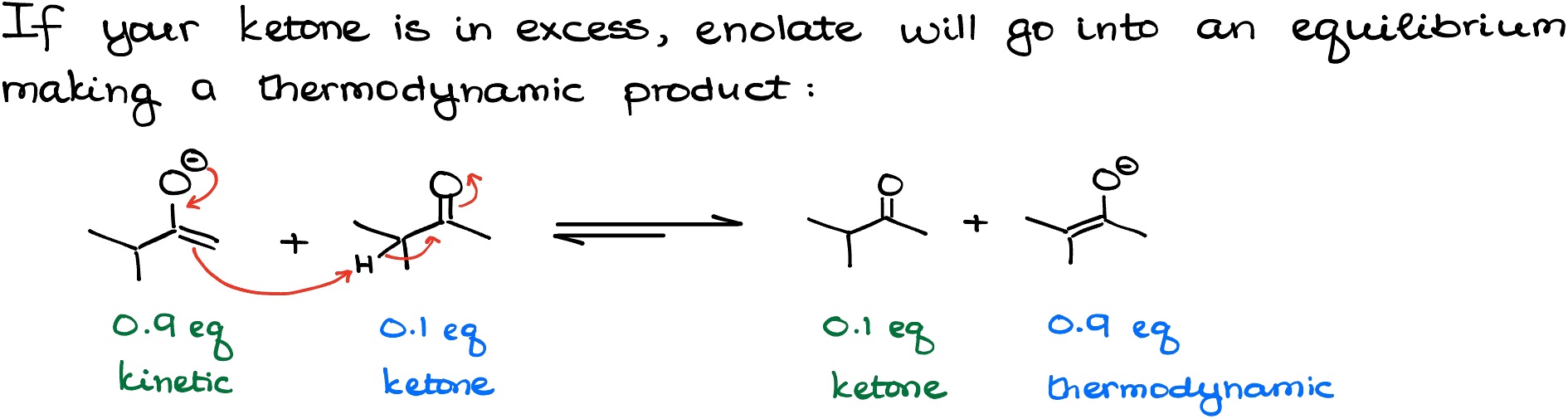
But as we run the surplus of the ketone by adding LDA to it dropwise, it allows the enolate to interact with the ketone reaching the state of equilibrium where the thermodynamic enolate is more abundant.
IMPORTANT! Not every instructor and textbook cover the use of LDA to make thermodynamic enolates. If your instructor does, they would write it something like 0.9 eq of LDA or 0.95 eq of LDA to indicate that the ketone is present in excess representing the thermodynamic conditions. If your instructor never talked about this, we’ll assume that LDA is taken in excess and we’re planning on making a kinetic enolate.
Using LDA to Make Kinetic Enolates
As I’ve mentioned above, LDA is a common base we’re going to see when we’re making a kinetic enolate. In this case, we’re going to run our enolization in so-called “kinetically controlled” conditions. What that means is that we’re going to do this reaction at lower temperature and we’re going to be adding our ketone to LDA to make sure that LDA is always in excess.
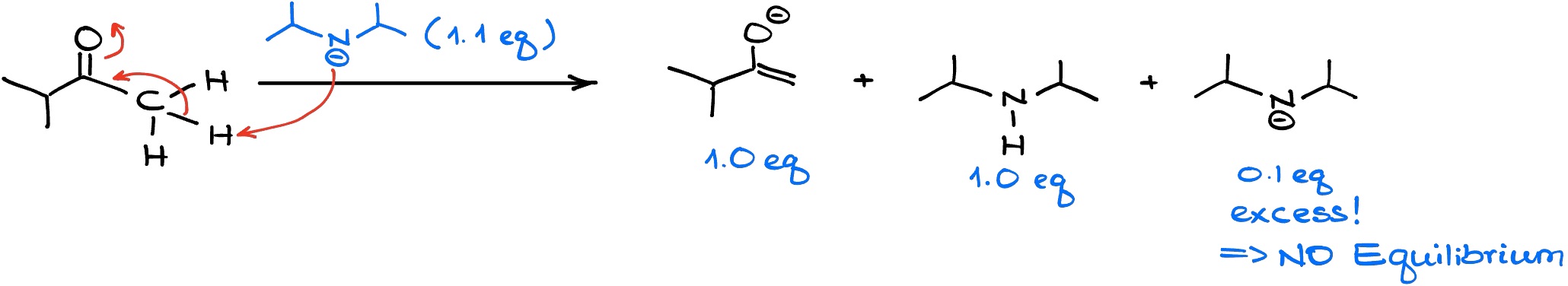
Since LDA is a strong and fast acting base, it will immediately snatch the proton off the more accessible ⍺-position. And since we don’t have any starting material to equilibrate with, we are going to end up with predominantly less substituted enolate.
But why does LDA prefer the less sterically hindered protons? The reasoning here is fairly simple, actually. If we look at the transition states for both possibilities, we’ll see that due to the LDA’s size, the transition state leading towards the formation of the more substituted (thermodynamic) enolate is higher in energy.
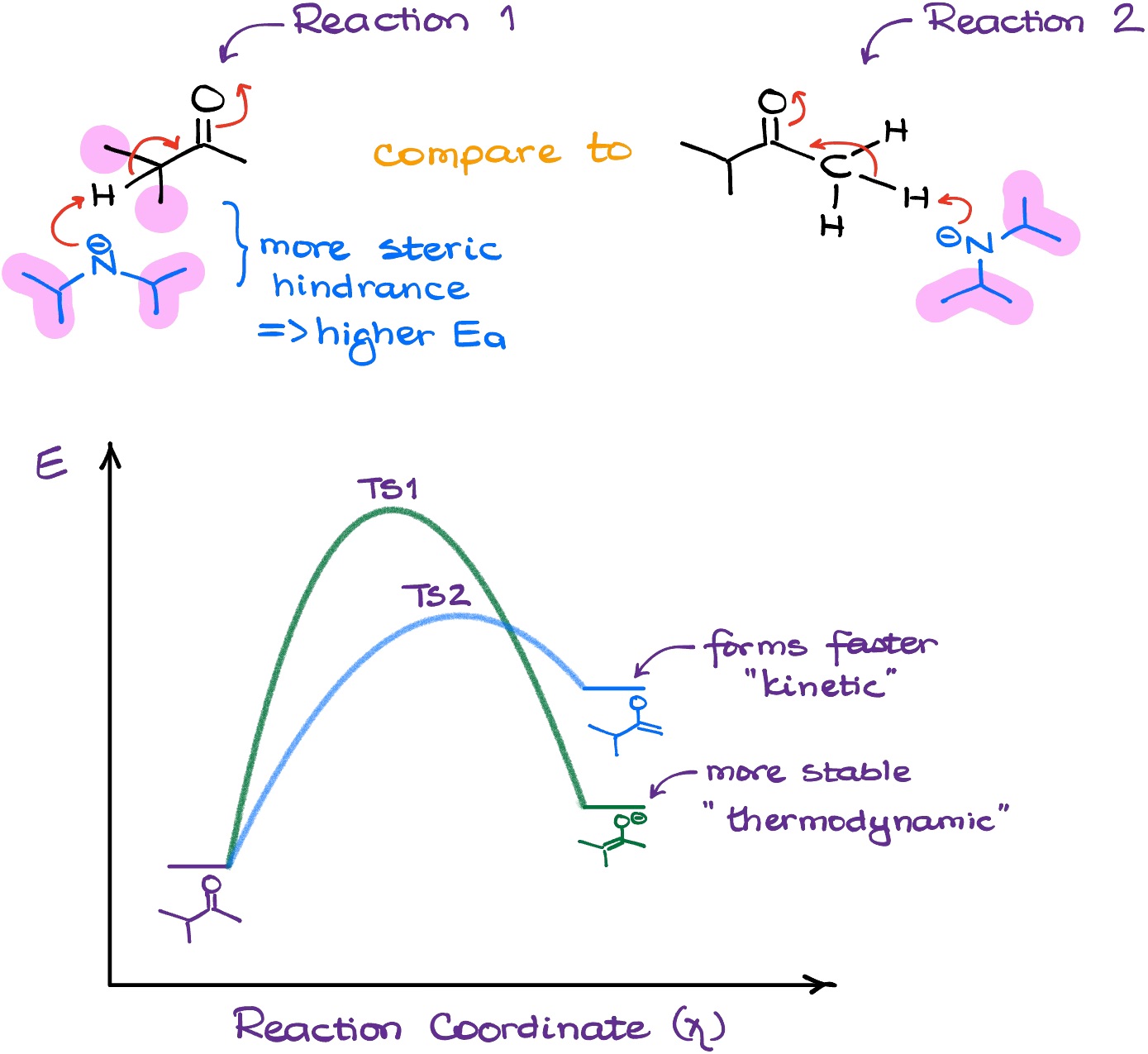
And since the other transition state is at lower energy, the formation of the kinetic enolate proceeds much faster. But because we do not allow for any equilibrium, once we get the kinetic enolate, there’s no going back.
Inf your instructor talked about using LDA for making thermodynamic enolates, they would say that now they are using 1.1 eq of LDA or something like this to indicate that we’re working in excess of LDA, and they intend to make the kinetic enolate.
It’s a lot of info, I know, so remember:
Thermodynamic Enolate: NaH or 0.9 eq LDA
Kinetic Enolate: excess or 1.1 eq of LDA
Also, if we’re using weaker bases, they also always make thermodynamic enolate. But due to the small equilibrium constants, those enolates are never going to be present in any large quantity for simple ketones.
Dicarbonyls Always Make Thermodynamic Enolates
Remember how 1,3-dicarbonyls are much more acidic than regular carbonyls? This is very relevant because for those species it doesn’t matter which base you use – you’ll always get your thermodynamic enolate pulling off the proton from between the carbonyls.
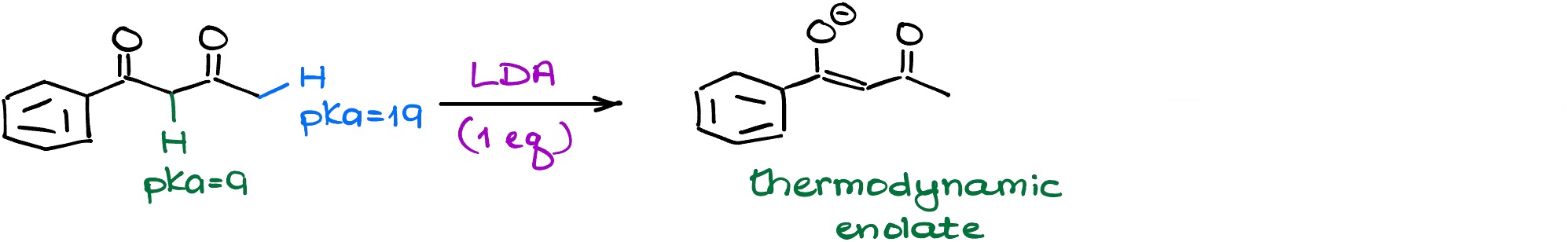
This is especially important to keep in mind if you’re planning to use your enolate for the further step in a multi-step synthesis. While not that many instructors try to purposefully “catch” students on this one, this is still a fair concept to test, and I have seen it on exams before.
Interestingly enough, you can double-enolize some dicarbonyls.
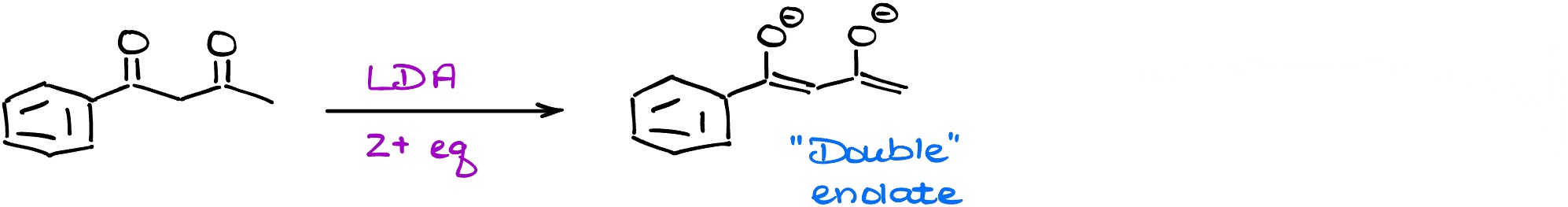
This can be a very nifty trick you might wanna remember for the future when you’re working on your synthesis homework or exam question.
