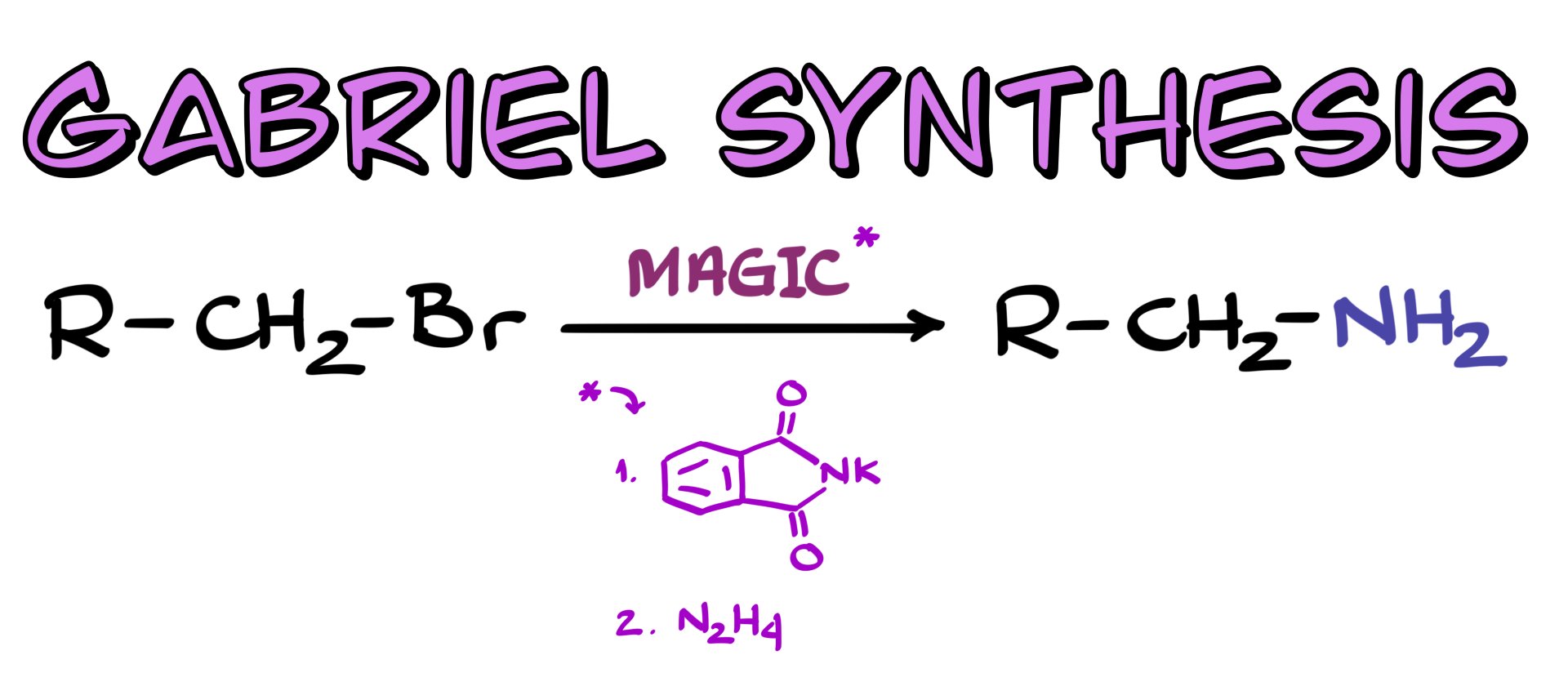
Gabriel Synthesis
In this tutorial, I want to talk about a very old yet somewhat useful synthetic procedure called the Gabriel Synthesis, which is a method for synthesizing primary amines. The general scheme of the reaction is fairly simple. We start with a primary alkyl halide and treat it with phthalimide, which gives us an intermediate structure. Then, we cleave this intermediate to release our primary amine—simple as that.
Gabriel Synthesis Mechanism
Now, there are a few things we need to discuss regarding the mechanism and the overall reaction. First, let’s talk about potassium phthalimide itself. This molecule is synthesized from phthalimide by treating it with potassium hydride, a very powerful base. Potassium hydride deprotonates the phthalimide, giving us the corresponding product where potassium acts as a counter-ion (which we don’t really care about), while hydrogen gas evolves. However, we don’t necessarily need to use such an extreme base like potassium hydride, which requires careful handling. The pKa of phthalimide is around 8, meaning it can be easily deprotonated with something as simple as potassium hydroxide without any issues. Within the scope of your class, you might see either method, and both are perfectly fine.
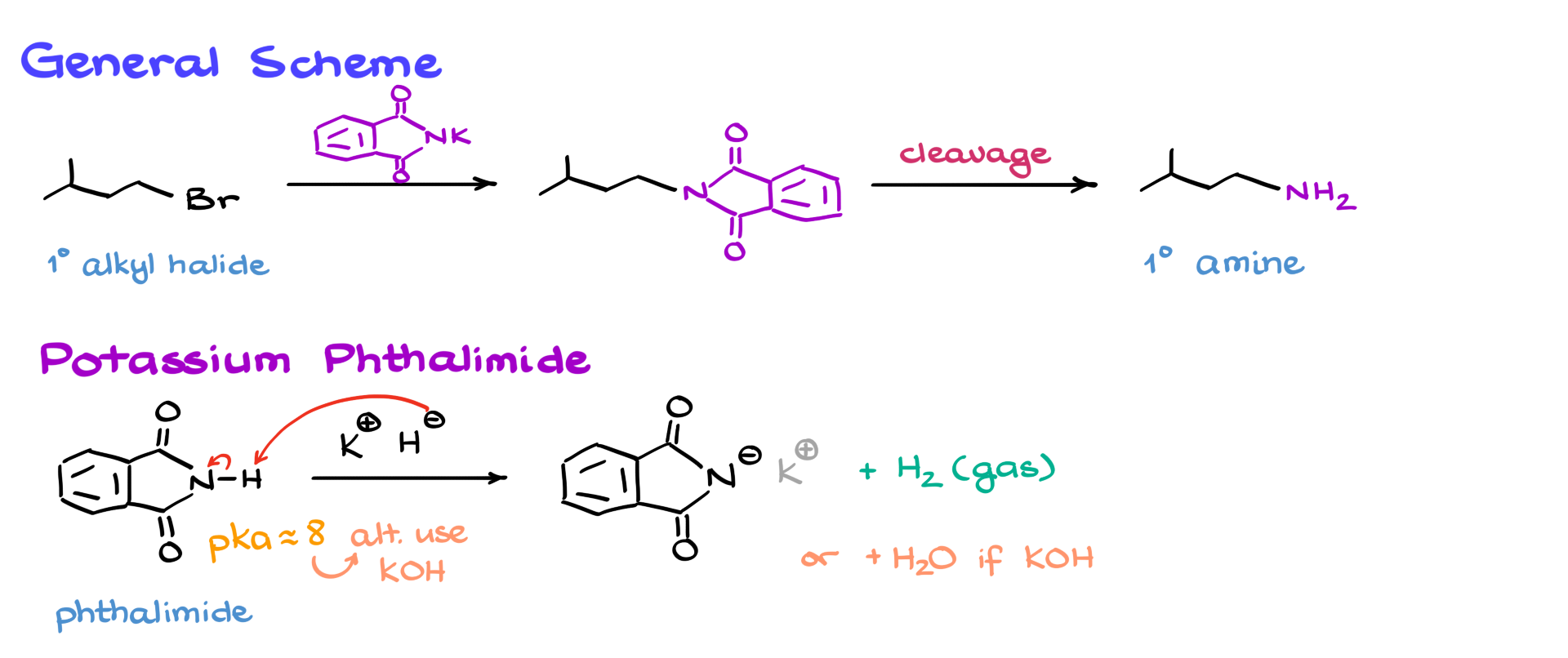
The core of this synthesis is an SN2 reaction between the primary alkyl halide and potassium phthalimide. Since potassium phthalimide is a very bulky nucleophile, the reaction is primarily limited to primary alkyl halides. If you try it with a secondary alkyl halide, the reaction will most likely fail. As expected in any other SN2 reaction, the nitrogen attacks the electrophilic carbon, displacing the leaving group (in this case, bromine) and forming a new carbon-nitrogen bond. I’ve highlighted this newly formed bond in the intermediate structure.
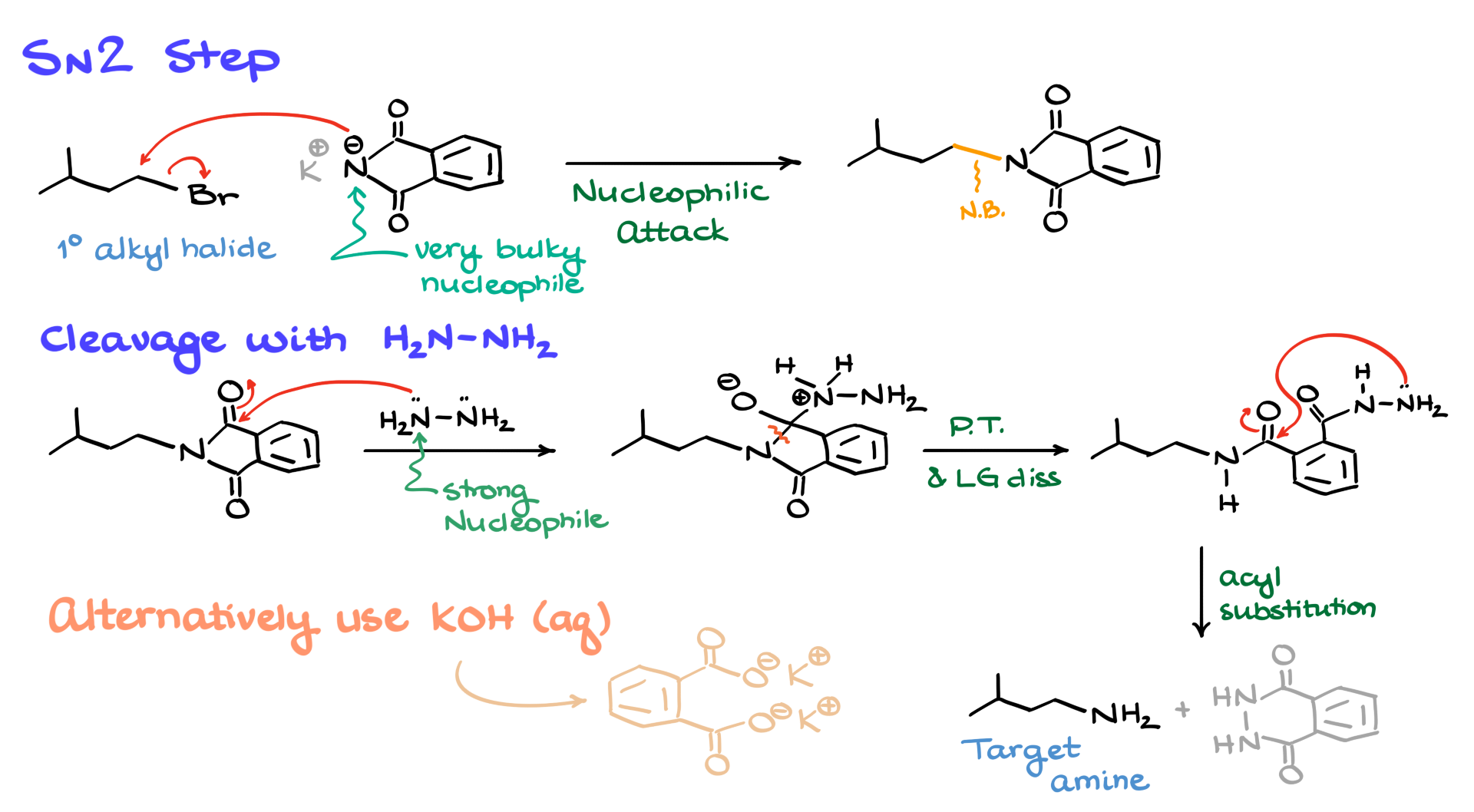
Once we have this intermediate, we proceed to cleave the phthalimide portion. The most common cleaving agent here is hydrazine. The first step involves a reaction between hydrazine and one of the carbonyl groups in the phthalimide. The nitrogen in hydrazine acts as a strong nucleophile, attacking the carbonyl and pushing electrons up onto the oxygen. This reaction occurs relatively easily because, even though an amide is not a very reactive electrophile, the strong nucleophilicity of hydrazine ensures the reaction proceeds.
From this step, we obtain an intermediate, and through a series of proton transfers and leaving group dissociations—following the usual steps of acyl substitution—the molecule eventually breaks at the carbon-nitrogen bond, forming another intermediate. At this point, an intramolecular interaction occurs where the other nitrogen in hydrazine attacks the second carbonyl. Again, after completing the standard acyl substitution steps, we end up with our target amine and a byproduct known as phthalic hydrazide.
One advantage of using hydrazine for cleavage is that phthalic hydrazide is a solid precipitate, which theoretically makes it easier to remove. However, in practice, isolating the pure product can be quite tricky, and the reaction can be challenging to work with. But since we’re dealing with this reaction on paper, we don’t have to worry about that too much, right?
An alternative cleavage method you might encounter in your class involves potassium hydroxide. In this case, the reaction undergoes basic hydrolysis of the amide, producing the potassium salt of phthalic acid, which is water-soluble and theoretically easier to remove. However, this method requires harsh conditions, often leads to poor yields, and can result in a number of side reactions.
Concluding Thoughts
Overall, the Gabriel Synthesis is not the best synthetic method. It tends to give poor yields, the reaction is highly temperamental, and working with it can be a real headache. While historically significant and still useful in some niche cases, this method is generally not used in modern organic synthesis. Instead, we typically rely on reductive amination, which is the premier method for amine synthesis today (and I have a tutorial on that as well).
That said, the Gabriel Synthesis is a common reaction tested in introductory organic chemistry courses, so you need to understand how it works and how to handle it in an exam setting.
