Hofmann Elimination
In this tutorial, I want to talk about yet another reaction bearing Hoffman’s name—the Hofmann elimination.

August Wilhelm von Hofmann was an extraordinarily prolific 19th-century German chemist, and his name pops up left and right when it comes to the classic chemistry of nitrogen-containing compounds. When it comes to the Hofmann elimination, the general idea starts with a primary amine. Unlike many other reactions we’ve seen in our course, this one is not just a single reaction but rather a sequence of steps leading to the final product.
Hofmann Elimination Mechanism
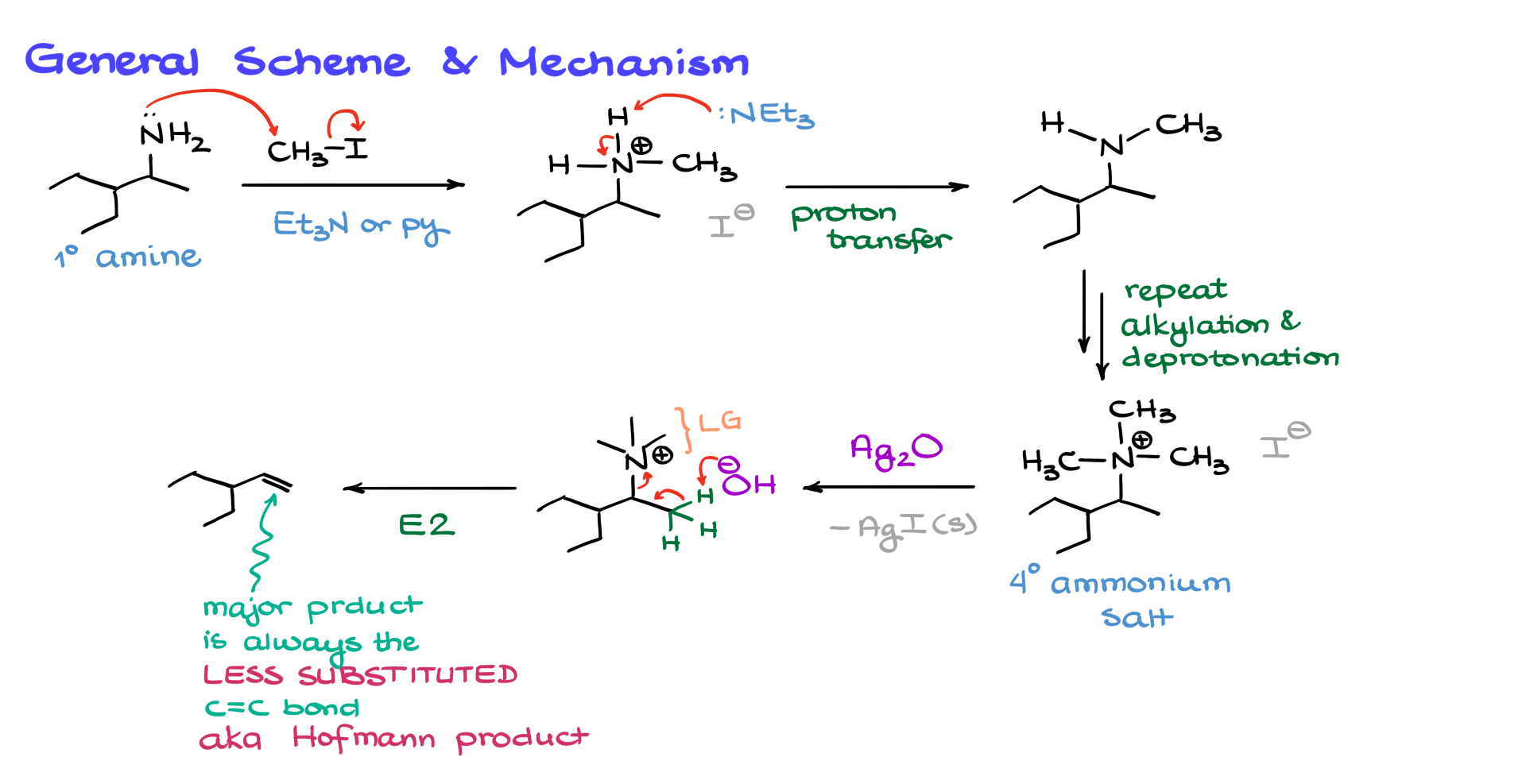
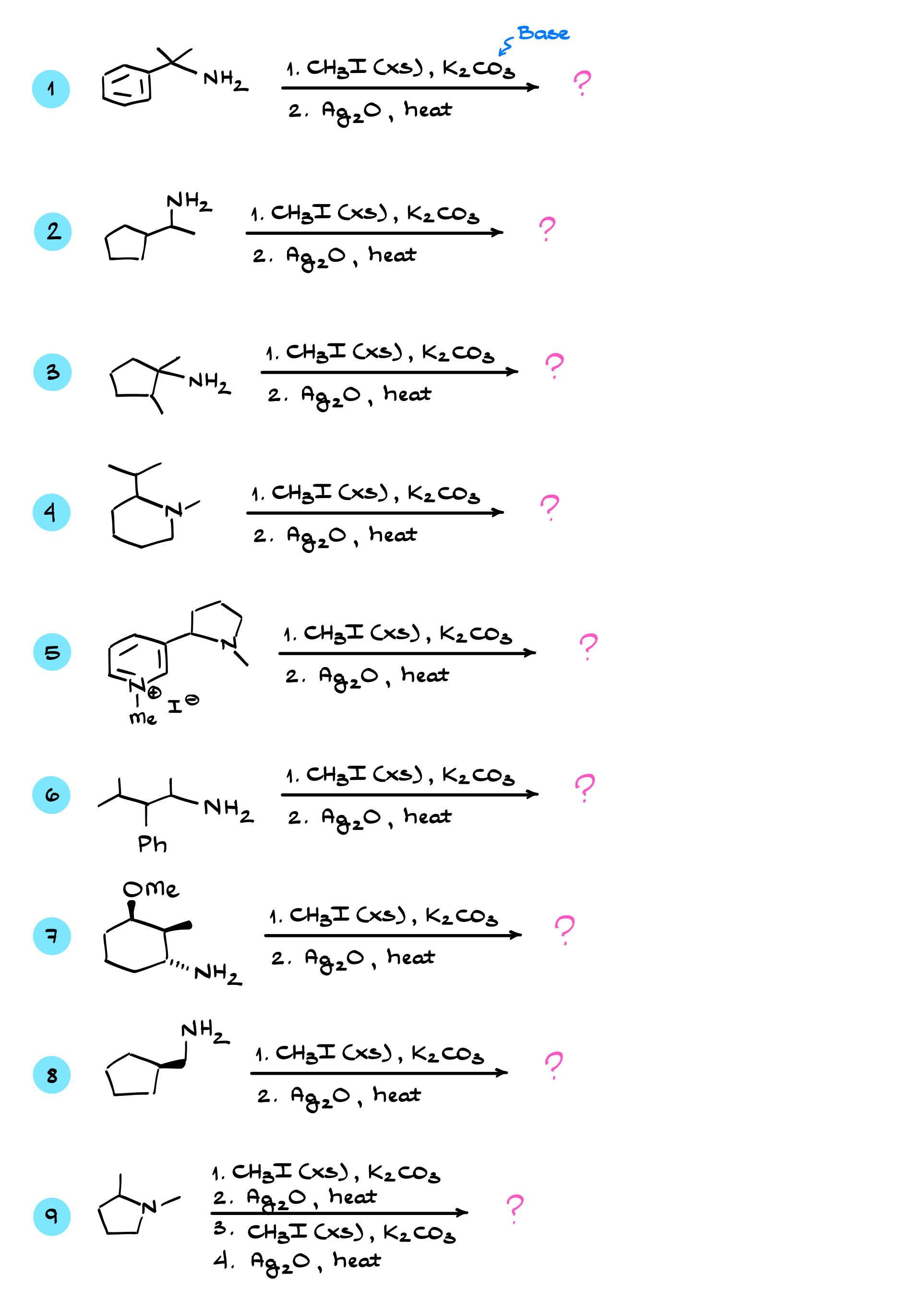
The first step in this sequence is the methylation of our nitrogen. Let’s look at how that happens step by step. The nitrogen atom here is a decent nucleophile, while the methyl group—the carbon in CH₃I (methyl iodide)—is a good electrophile. The nitrogen will come in and attack the carbon of the methyl iodide, kicking out the iodine, which acts as the leaving group, giving us the following intermediate. This reaction is typically done in the presence of a basic solvent like triethylamine or pyridine, though we sometimes don’t explicitly write it down. The role of the solvent is to come in and deprotonate that intermediate—essentially, the nitrogen pulls off a proton, giving us a free amine.
As long as we have a free amine with an electron pair on nitrogen, the methylation can continue happening. This process repeats over and over again until we get a quaternary ammonium salt. Since the nitrogen atom is positively charged, there will be a negatively charged counterion, which in this case is I⁻. This counterion doesn’t really matter much for us, but we’ll keep showing it for now.
Silver Oxide Role
Once no further alkylations are possible, we switch our reagents, introducing silver oxide. Typically, this is done in an aqueous solution with some added ammonia to dissolve the silver oxide—something similar to Tollens’ reagent. However, there are multiple variations of how this reaction is performed, and silver oxide can be used under different conditions.
The role of silver oxide is to replace iodine (I⁻) with hydroxide (OH⁻), forming silver iodide as a precipitate. The exact mechanism of this exchange isn’t our main concern—we just need to remember that I⁻ is replaced by OH⁻. The presence of OH⁻ is crucial because hydroxide itself is a base, and the nitrogen with its three methyl groups can now act as a leaving group. Since we have both a base and a leaving group, elimination can take place.
Elimination Step
At this stage, we need to look at the β-hydrogens relative to the leaving group. The OH⁻ will come in, pull one of these β-hydrogens off, and push out the leaving group, forming a double bond. The product of the Hofmann elimination is always an alkene, and a key point to remember is that we obtain the less substituted carbon-carbon double bond. This is known as the Hofmann product, which you might recall from studying elimination reactions. This formation of the less substituted product is a feature, not a bug.
Regioselectivity of the Hofmann Elimination
You might wonder why exactly we get the less substituted product. To understand that, let’s analyze the elimination step more closely. The reaction could theoretically yield two different products. If we remove a β-hydrogen from one side, we get the more substituted alkene (the Zaitsev product). If we remove a β-hydrogen from the other side, we get the less substituted Hofmann product.
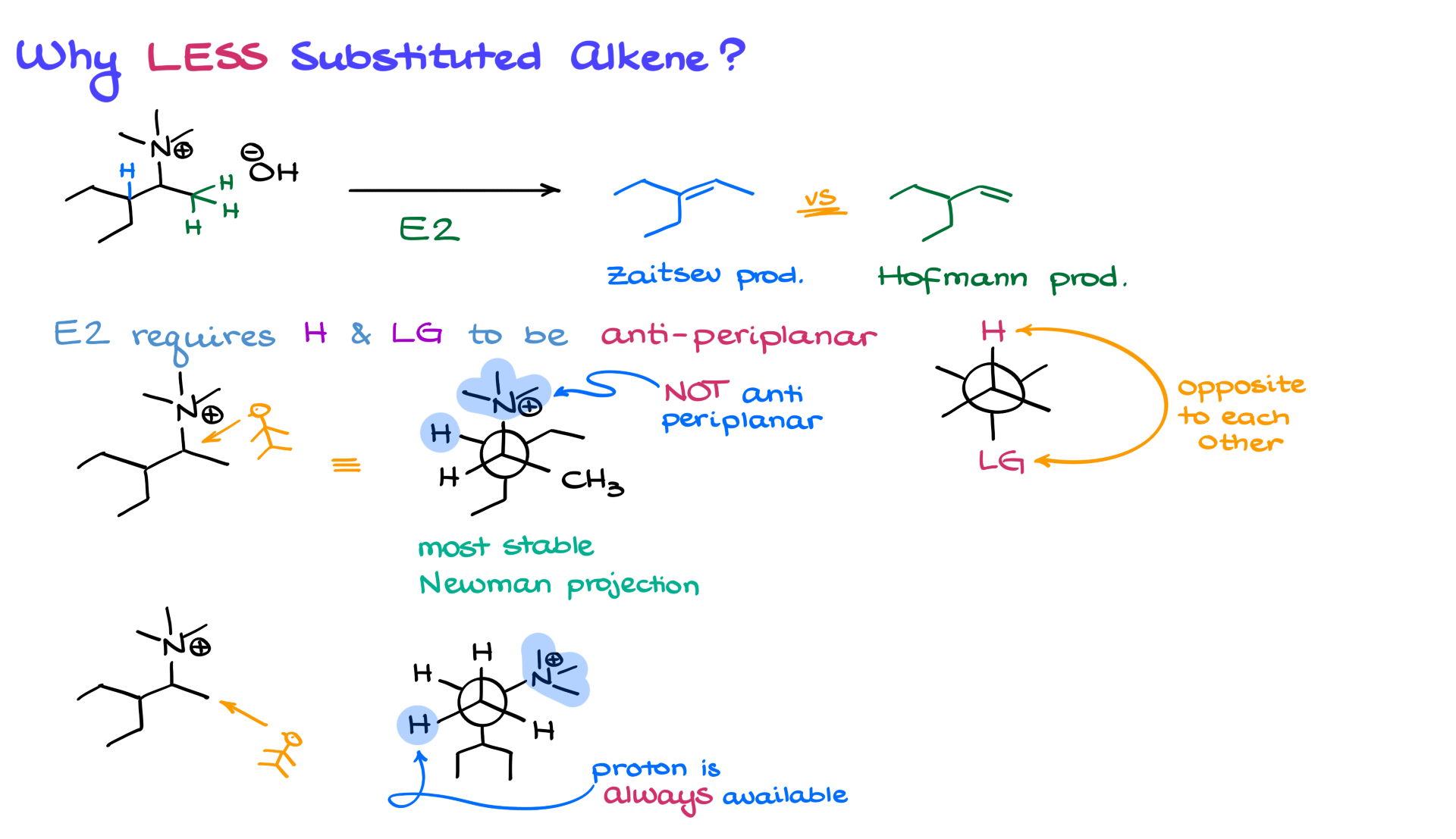
The key factor here is the E2 reaction mechanism, which requires the hydrogen and leaving group to be anti-periplanar—meaning they must be positioned opposite to each other in the same plane. If we draw a Newman projection of our molecule, we see that only one of the β-hydrogens is perfectly aligned with the leaving group in this anti-periplanar fashion. The bulkiness of the trimethylamine group makes conformational changes difficult, so the molecule remains in the orientation where elimination favors the Hofmann product. The bottom line is that elimination always occurs from the less substituted β-position because it is the more accessible conformation.
Whenever you’re working with these reactions, always check which β-position has fewer substituents—that’s where the elimination will occur. Let’s illustrate this with a couple of examples.
Example 1
In the first example, we start with the following molecule.
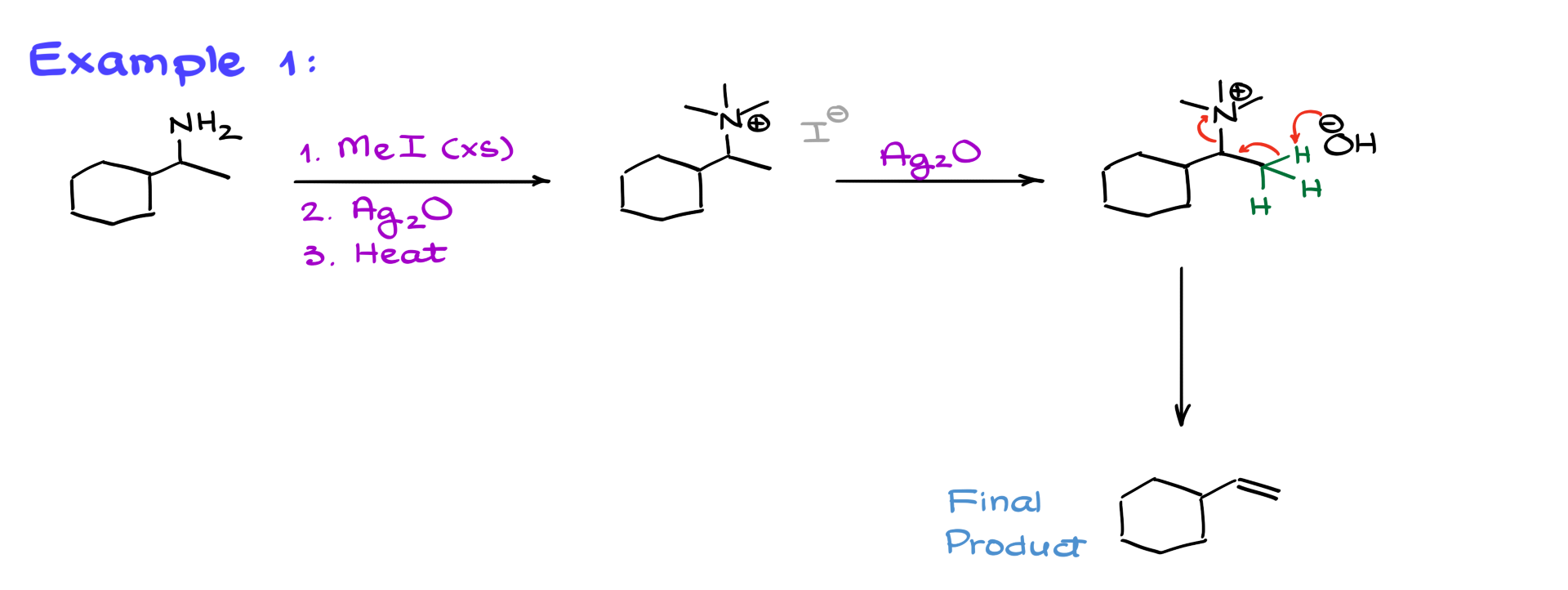
The first step is exhaustive methylation, which gives us the quaternary ammonium salt. The next step is treatment with silver oxide, replacing I⁻ with OH⁻. From this point, elimination could occur at two positions: a less substituted β-carbon on the right and a more substituted one on the left. Since we always go with the less substituted side, elimination happens on the right, producing the final alkene.
Example 2
In another example, we again begin with a an amine.
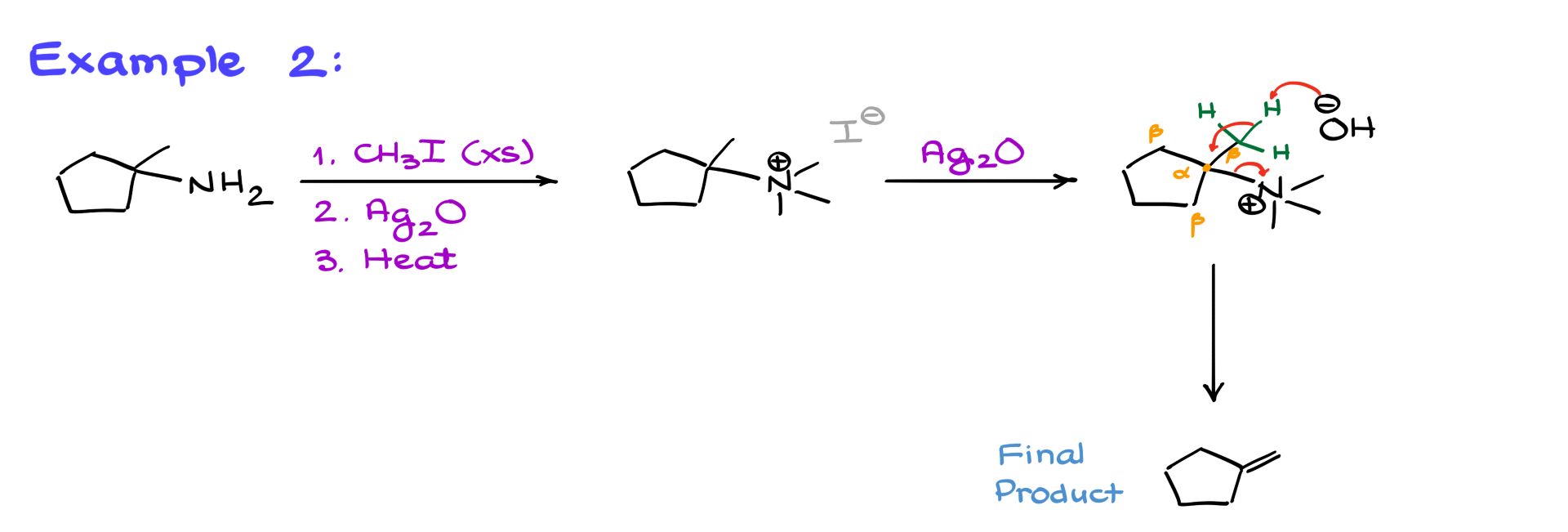
The first step is exhaustive methylation, forming the quaternary ammonium salt. Then, silver oxide replaces I⁻ with OH⁻, giving us an intermediate. We identify the α-position (where the nitrogen is attached) and the β-positions. The least substituted β-position is at the top right, so we draw the hydrogens there and show OH⁻ removing one, leading to elimination and formation of the final product. No matter where nitrogen is in the molecule, you simply check the surrounding β-positions, find the least substituted one, and proceed accordingly.
Example 3
For the last example, let’s consider a trickier case, often seen in exams.
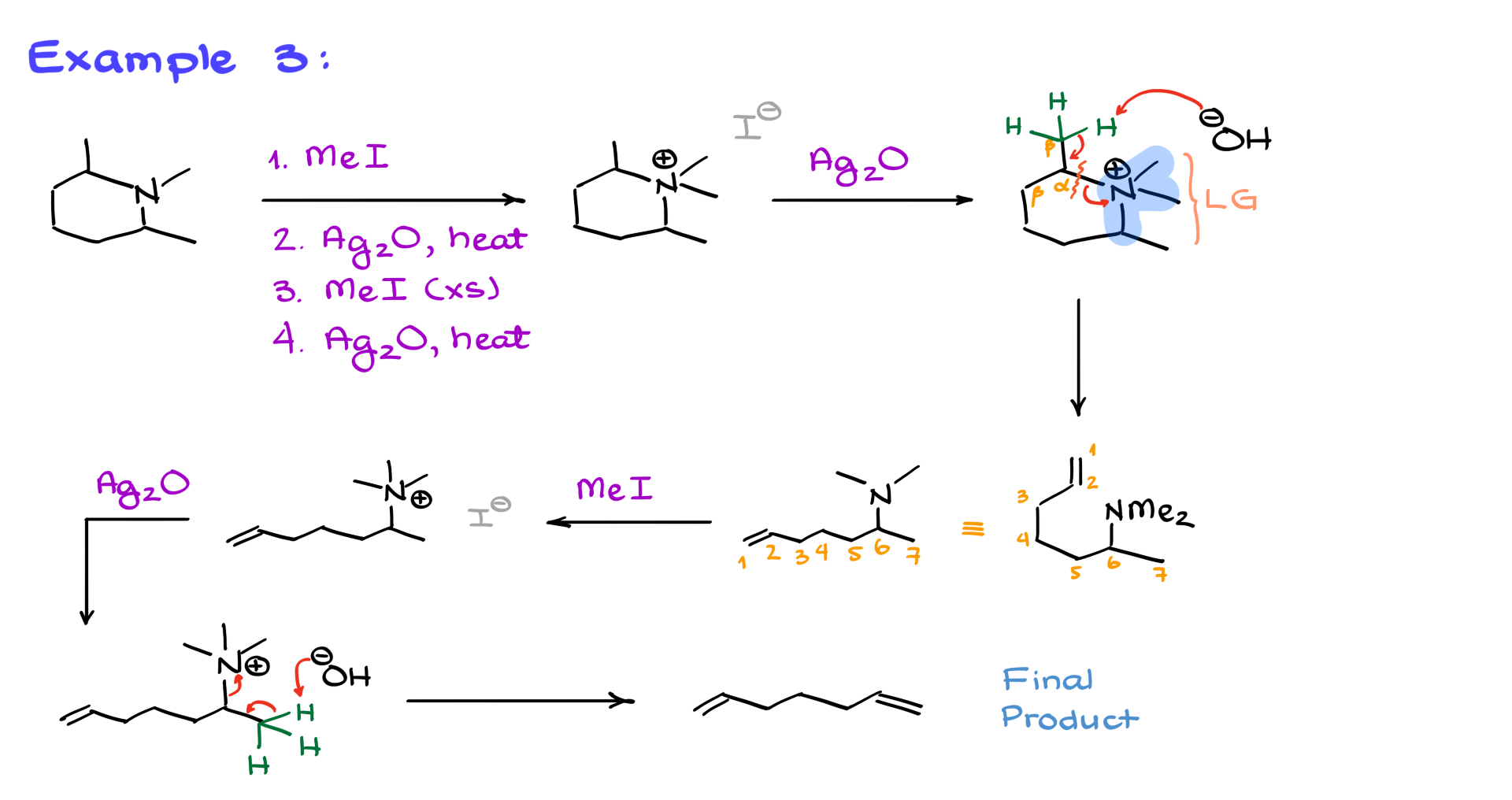
Here, we see methyl iodide and silver oxide followed by heat—twice. This suggests that Hofmann elimination occurs twice in succession. Step one is exhaustive methylation, forming the quaternary ammonium salt. Step two is treatment with silver oxide, replacing I⁻ with OH⁻. At this point, we identify the least substituted β-position and eliminate accordingly. But here’s the twist—after elimination, the nitrogen is still present as part of the molecule, meaning it can undergo a second round of Hofmann elimination.
We methylate again, forming another quaternary ammonium salt. Silver oxide replaces I⁻ with OH⁻, and we repeat the elimination process, identifying the least substituted β-position once more. Since in this case, the nitrogen is part of a ring system, elimination actually results in ring opening, producing a linear alkene with two double bonds as the final product.
As you can see, the Hofmann elimination isn’t a difficult reaction as long as you carefully follow the steps. Now, you have another elimination reaction in your arsenal of synthetic tools, ready to be used in your next multi-step synthesis when you need to create something fancy.
Practice Questions
Would you like to see the answers and check your work? Become a member today or login if you’re already a member and unlock all members-only content!