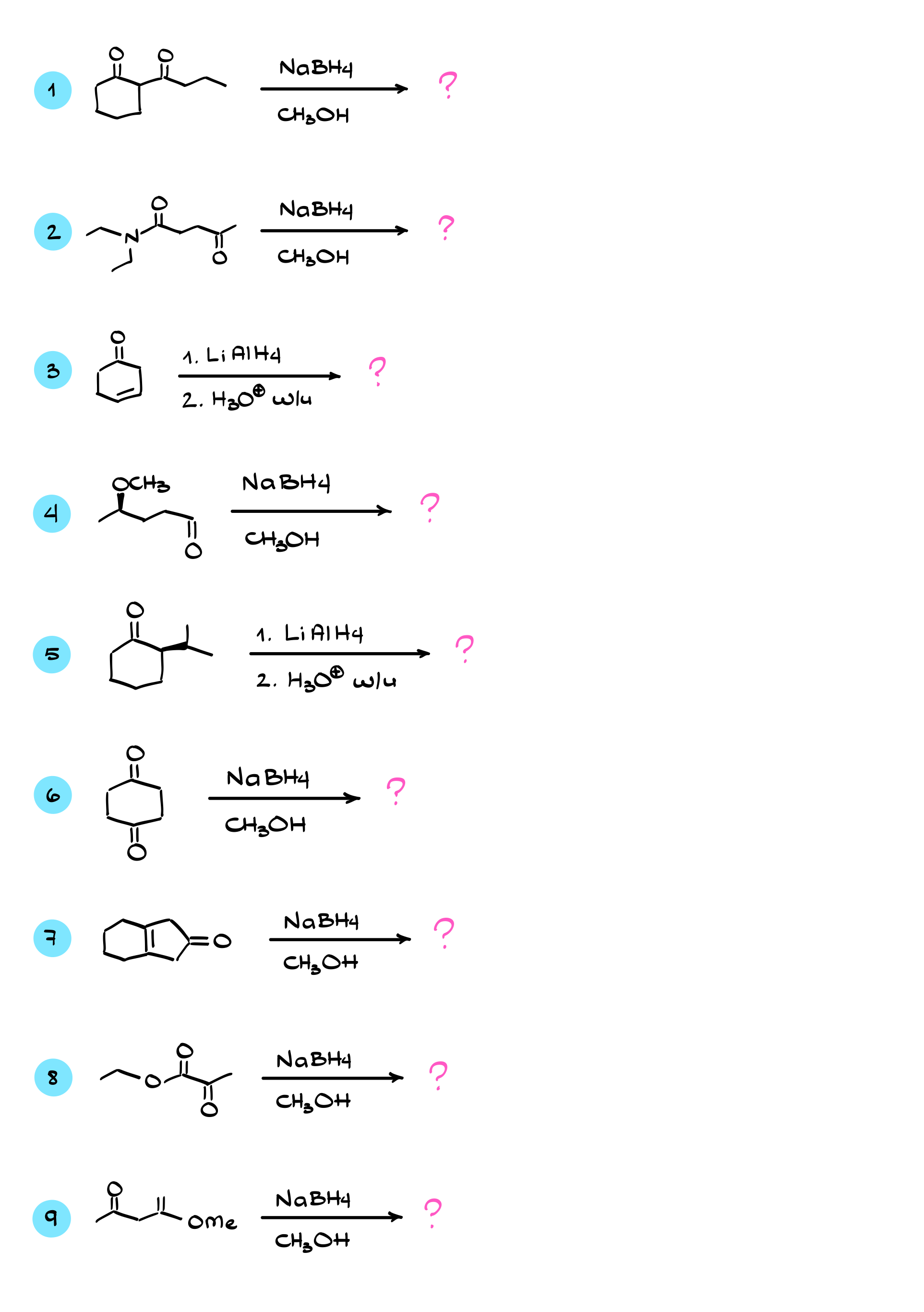
Reduction of Aldehydes and Ketones with Complex Hydrides
In this tutorial, I want to talk about the reduction of carbonyls, specifically aldehydes and ketones, using complex hydrides like sodium borohydride and lithium aluminum hydride. Let’s start by looking at the mechanism of this reaction, beginning with sodium borohydride.
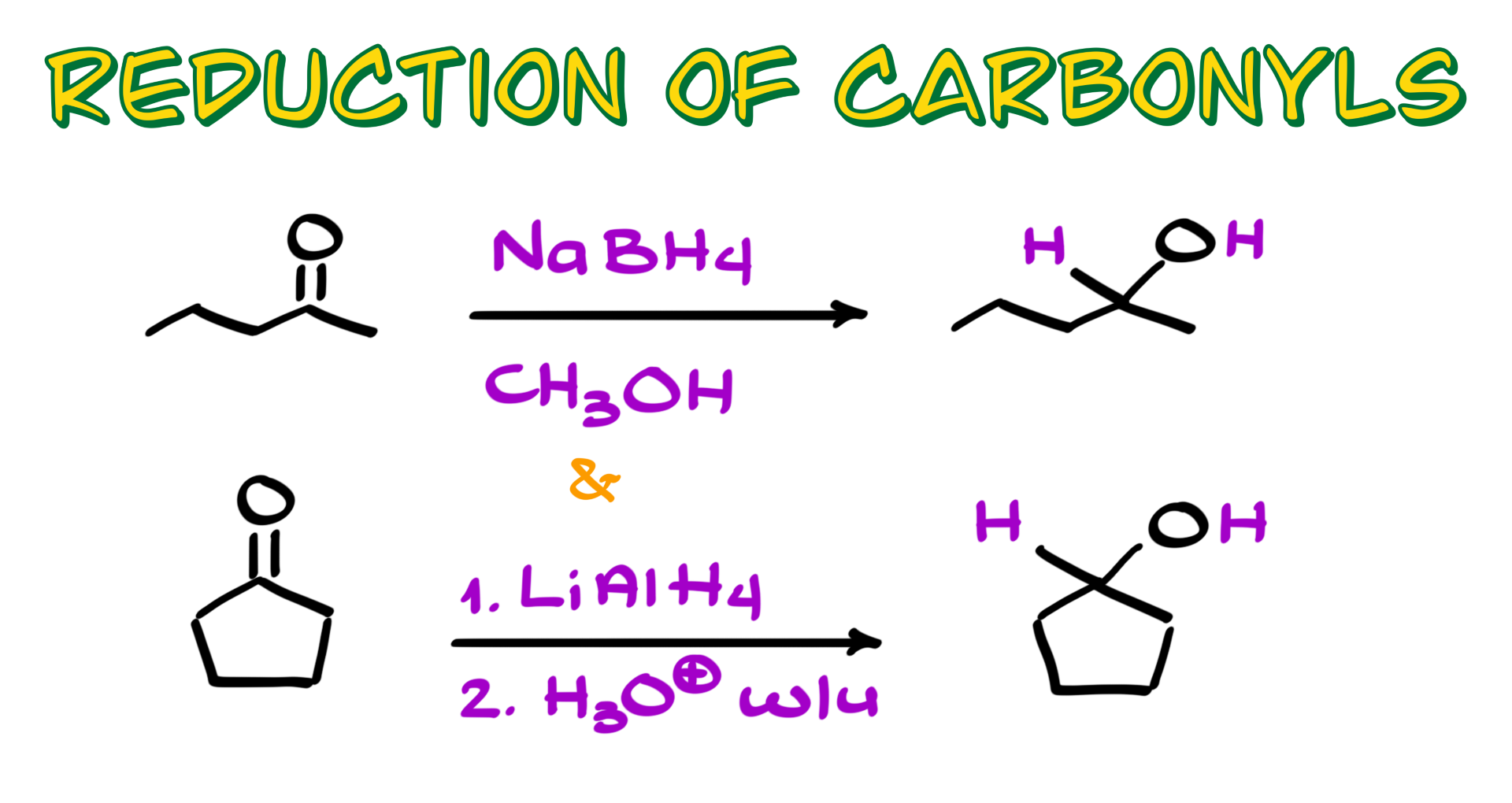
Mechanism of the Reduction of Carbonyls
First, I want to clarify a few things. When you see methanol in these reactions, it primarily serves as a solvent and a proton source. On the other hand, sodium borohydride is our reducing agent. To get started, I’ll draw sodium borohydride. The sodium ion is just a counterion, so we don’t really focus on it. What we care about is the BH₄⁻ ion, which acts as our reducing agent.
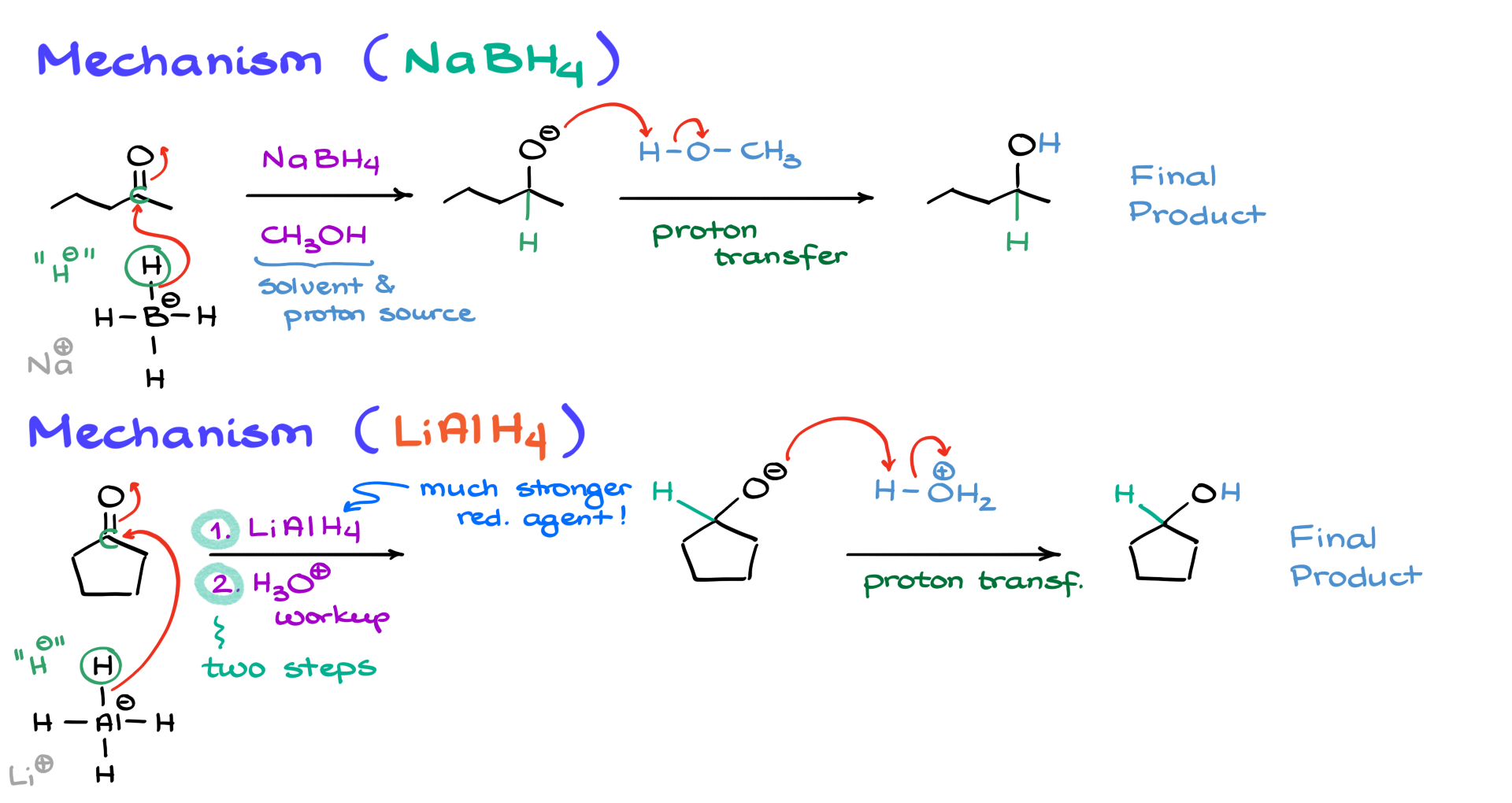
The first step in this reaction involves a nucleophilic attack from the borohydride onto the carbonyl. Essentially, we are forming a new bond between the hydrogen and the carbon of the carbonyl. You can think of borohydride as a source of nucleophilic H⁻. It’s important to note that we are not forming a bond between carbon and boron—this is not a hydroboration reaction like the one you’ve learned for alkenes.
After the nucleophilic attack, a new bond forms between the hydrogen and the carbonyl carbon, generating a negatively charged oxygen. At this point, we have an alkoxide intermediate. Since we don’t keep alkoxides around, the methanol present in the reaction provides the necessary proton for proton transfer. The oxygen grabs a proton from methanol, giving us our final product—an alcohol. Pretty straightforward, right?
Now, let’s discuss lithium aluminum hydride. The mechanism is quite similar, but there are some key differences. First, lithium aluminum hydride is a much stronger base and a more reactive nucleophile, meaning it provides H⁻ more aggressively. Because of this, we must carry out this reaction in two steps. Unlike sodium borohydride, which reacts slowly with methanol, lithium aluminum hydride reacts explosively with any proton source. That’s why we perform the reduction first and then add an acid in a separate step.
In the first step, lithium aluminum hydride reacts with the carbonyl much like sodium borohydride does. The hydride attacks the carbonyl carbon, forming a new carbon-hydrogen bond and generating a negatively charged alkoxide intermediate. The second step is the acidic workup, where the oxygen picks up a proton from an acid, ultimately giving us the final product—an alcohol.
So, if the mechanisms and products are the same, does that mean lithium aluminum hydride and sodium borohydride are interchangeable? For aldehydes and ketones, yes. However, their reactivity towards carboxylic acids and their derivatives is different, but that’s a topic for another tutorial.
Now, let’s look at some examples.
Example 1
For the first example, we have cyclopentane carbaldehyde reacting with sodium borohydride in methanol.
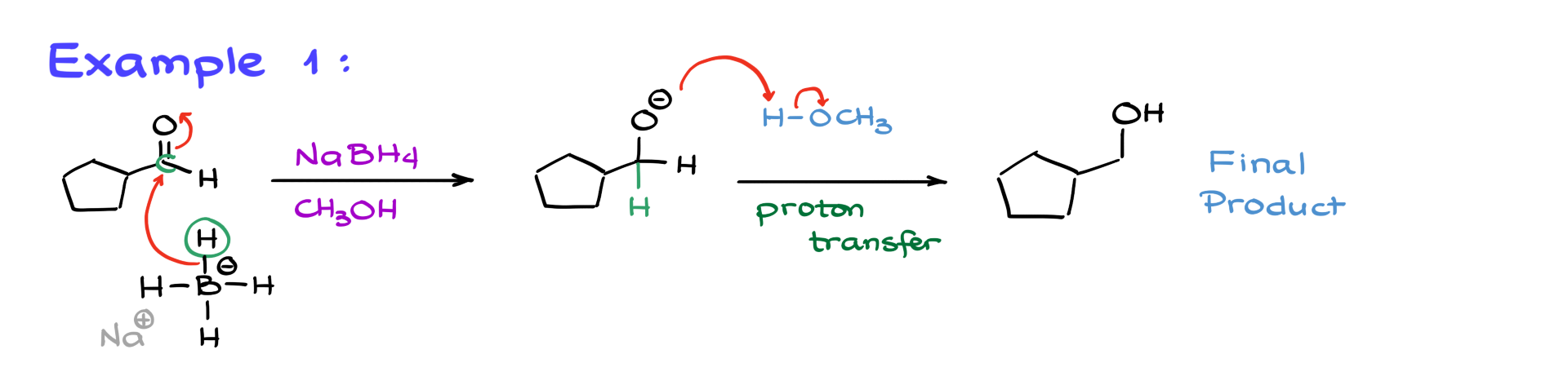
We follow the same mechanism—borohydride attacks the carbonyl, forming a new carbon-hydrogen bond, which results in an alkoxide intermediate. This intermediate then grabs a proton from methanol, yielding the final product, a primary alcohol. Simple, right?
Example 2
Next, let’s consider a more complex case—a ketone reacting with lithium aluminum hydride followed by an acidic workup.
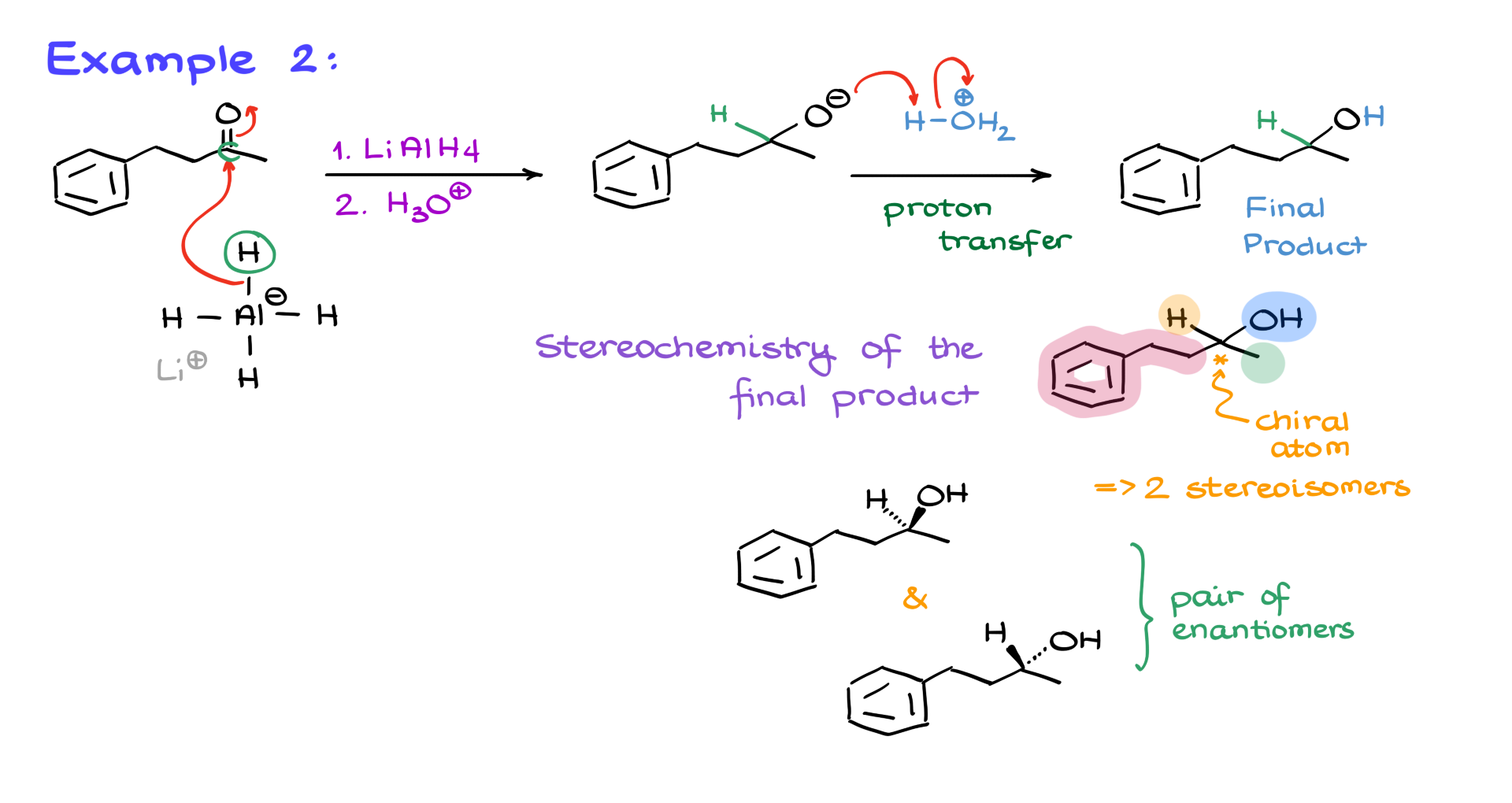
Again, the first step involves the nucleophilic attack by the hydride, creating an alkoxide intermediate. The second step is the protonation of the alkoxide by an acid, leading to the formation of a secondary alcohol.
One important point here is stereochemistry. When you add a hydrogen to a carbonyl, the resulting carbon can become a stereocenter. In this case, we have a chiral center with four different groups attached, meaning we obtain two different stereoisomers. Some instructors may not emphasize this, but others will expect you to recognize it. You might be asked to determine the major product or describe the relationship between the stereoisomers. In this example, we get two molecules: one where the OH group is pointing towards us and another where it’s pointing away. These are a pair of enantiomers.
Example 3
However, don’t assume that every secondary alcohol will result in enantiomers. For example, if we reduce the following cyclic ketone using sodium borohydride, the stereochemical outcome can be different.
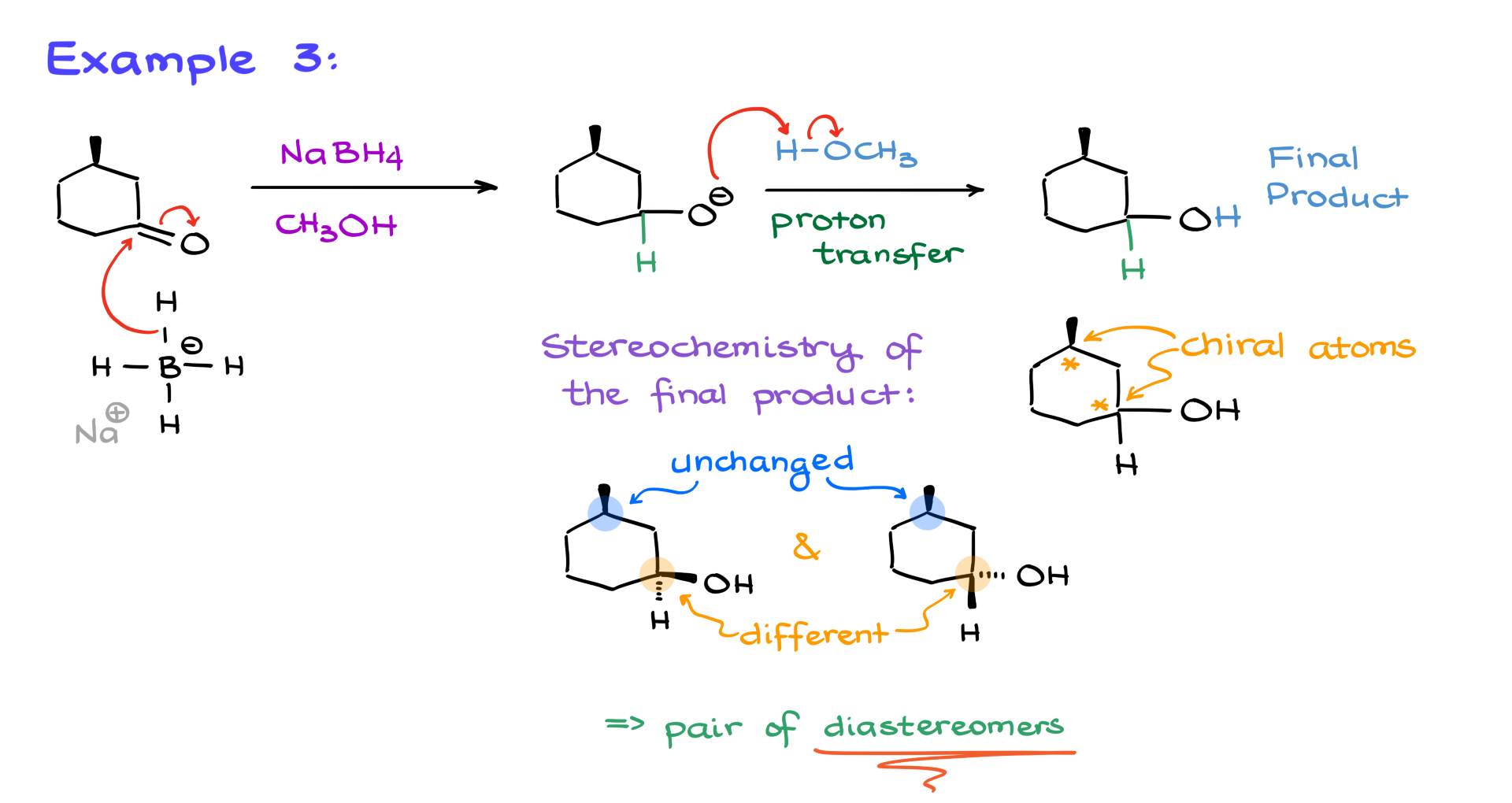
If we analyze the stereochemistry carefully, we may find that one of the stereocenters remains unchanged while the other one can adopt two different configurations. This means we end up with diastereomers instead of enantiomers. So, always analyze the actual products instead of making assumptions.
Example 4
Now, let’s discuss a trickier case.

Suppose we have a molecule where the oxygen in the alkoxide intermediate also acts as a nucleophile. If there is an electrophilic carbon with a leaving group present in the same molecule, an intramolecular reaction can occur. Since intramolecular reactions happen much faster than intermolecular ones, the oxygen attacks the electrophilic carbon, forming a new bond and generating a five- or six-membered ring. These reactions happen so efficiently that if the possibility to form a stable ring exists, it is likely to occur. This is something to watch out for on exams.
Example 5
Finally, let’s look at an example I call “catch them all” (just like Pokémon).

In this case, we have a molecule containing both a ketone and an aldehyde. Since lithium aluminum hydride and sodium borohydride cannot effectively distinguish between aldehydes and ketones in terms of reactivity, both functional groups will be reduced. This means we will obtain a diol as our final product.
If stereochemistry is involved, we might end up with multiple chiral centers. This could lead to multiple stereoisomers—not just a pair of enantiomers or diastereomers, but possibly four or even eight different products. In cases like this, instructors often tell students to ignore stereochemistry unless specifically asked to draw all possible products.
That wraps up today’s tutorial! Hopefully, this breakdown makes it easier to understand the reduction of aldehydes and ketones using sodium borohydride and lithium aluminum hydride. Keep practicing, and be mindful of stereochemistry, reactivity differences, and possible intramolecular reactions.
Practice Questions
Would you like to see the answers and check your work? Become a member today or login if you’re already a member and unlock all members-only content!