Oxidation States in Organic Molecules
In this tutorial, I want to talk about oxidation states. So first of all, what exactly is an oxidation state? The oxidation state, or oxidation number if you like, is a hypothetical charge that an atom would have if the bonding electrons were assigned to the more electronegative atom in each bond. Essentially, we are treating each bond as if it were an ionic bond rather than a covalent bond. But there is a very important distinction between oxidation states and charges—they are not the same thing.
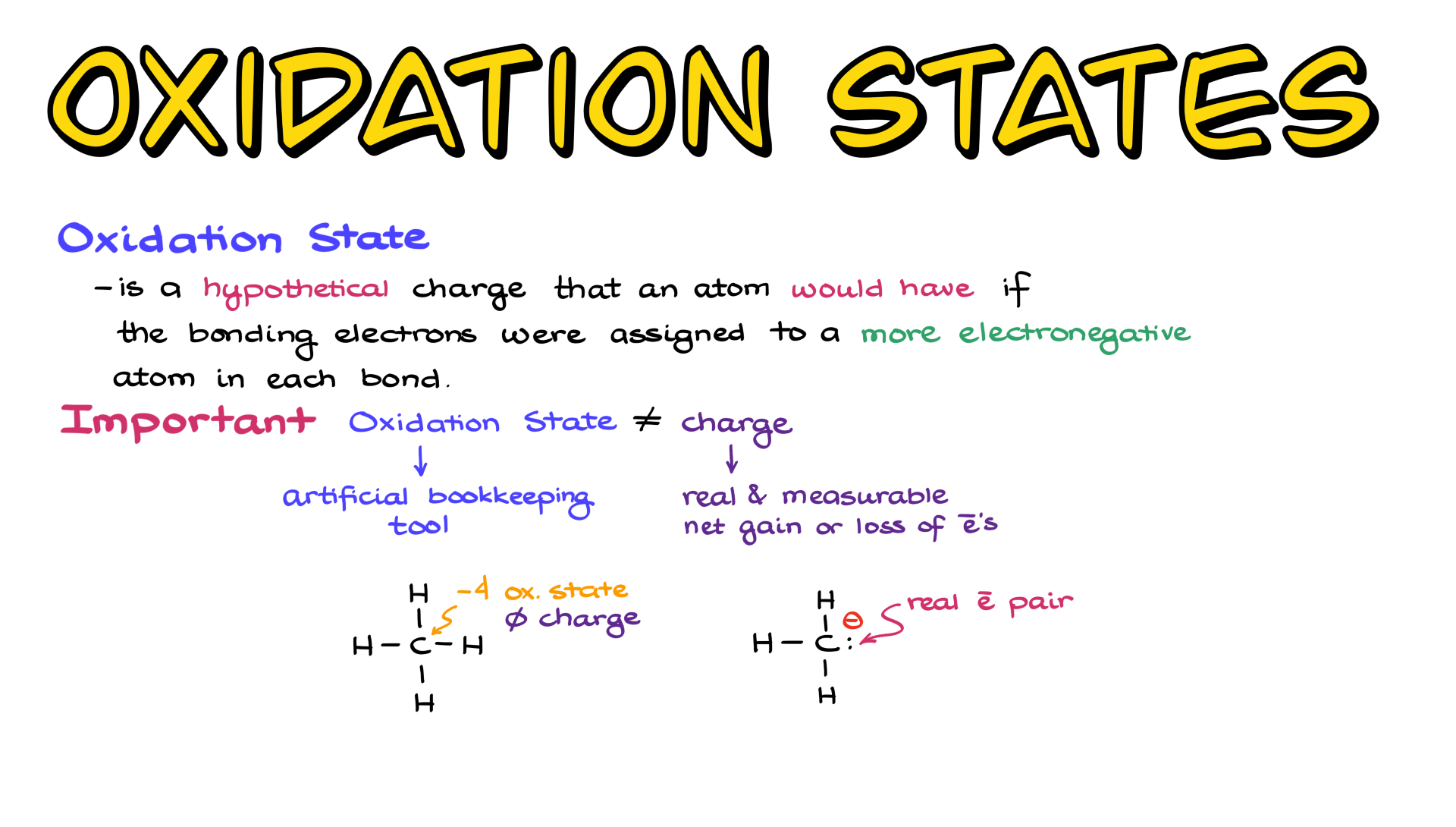
When it comes to charge, it is a real and measurable net gain or loss of electrons in a molecule. For instance, if I look at the methyl anion, CH₃⁻, we have a very real electron pair on carbon, and because of that, carbon has an excess of electron density, giving it a negative charge. However, oxidation states or oxidation numbers are artificial bookkeeping tools—they have nothing to do with reality.
How to Quickly Calculate Oxidation States of Atoms in Organic Molecules
So if I look at something like methane, the oxidation state of carbon in methane is -4, but the actual charge of carbon in methane is 0.
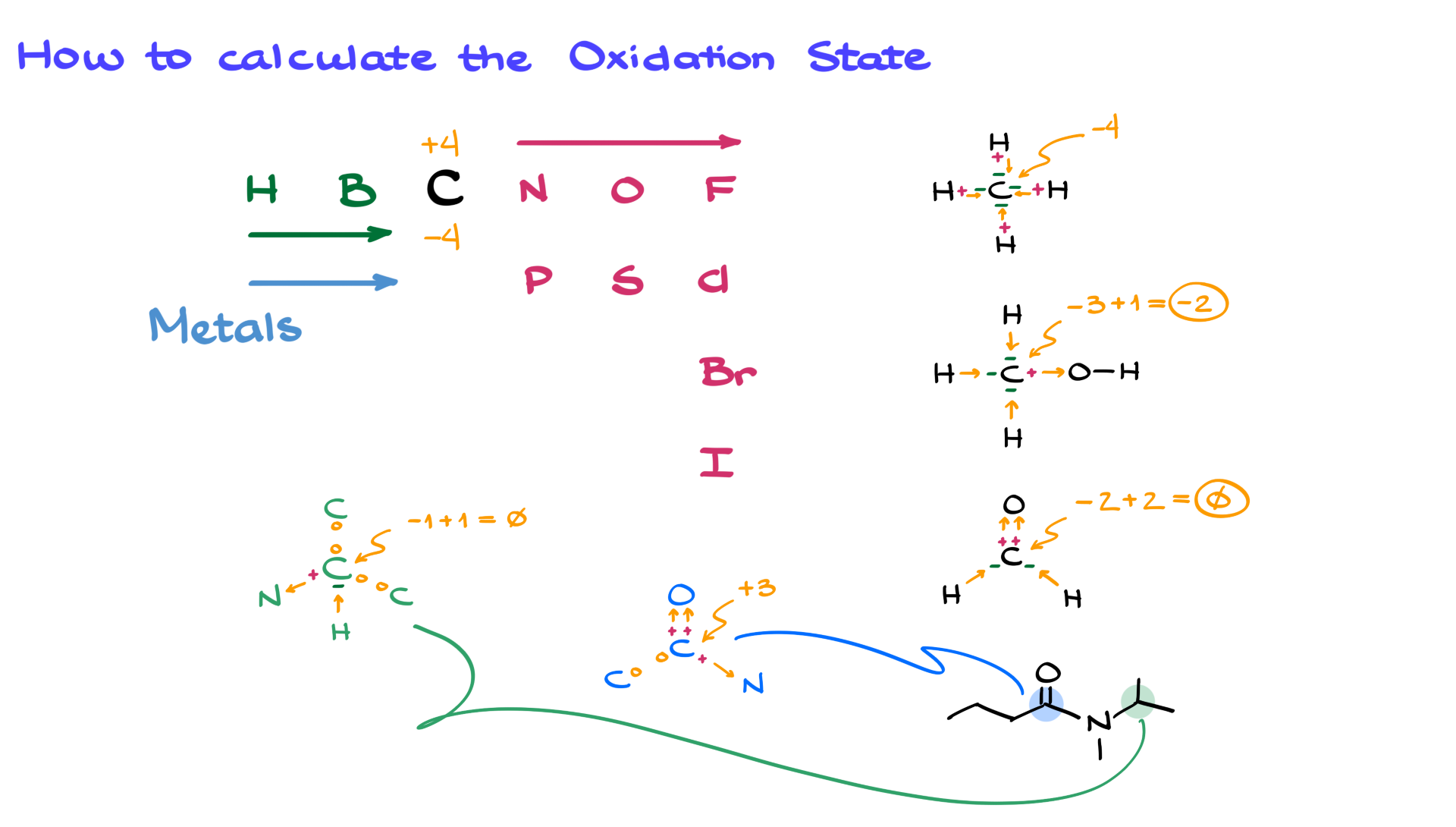
Now that we’ve established the difference between oxidation states and charges, let’s look at how we calculate oxidation states. In organic chemistry, we are primarily interested in the oxidation state of carbon. The oxidation state of carbon can range from -4 to +4, depending on what it is bonded to. Normally, we would look up the electronegativities of the elements connected to carbon to determine how the bond is polarized. But luckily, there is a simple trick so you don’t have to remember the exact numbers.
Nonmetals to the left of carbon in the periodic table, like hydrogen and boron, have lower electronegativity than carbon, meaning they push electron density toward carbon. This makes carbon more negative when bonded to these elements. On the other hand, nonmetals like nitrogen, oxygen, fluorine, and sulfur have higher electronegativity than carbon, so they pull electron density away, making carbon more positive. Additionally, all metals have lower electronegativity than carbon and will push electron density onto carbon, making it more negative in a carbon-metal bond.
Armed with this information, let’s apply it to an example. Take methane—by looking at the periodic table, we see that each hydrogen is pushing electron density toward carbon. Since there are four hydrogen atoms, the oxidation state of carbon in methane is -4.
Now, let’s look at something slightly more complex, like methanol. Here, hydrogens are still pushing electron density onto carbon, but oxygen is pulling electron density away from carbon. Each carbon-hydrogen bond makes carbon more negative, while the carbon-oxygen bond makes carbon more positive. When we combine these effects, we find that the oxidation state of carbon in methanol is -2.
We use the same principle when dealing with double or triple bonds, counting each bond separately. In a molecule with a carbon-oxygen double bond, for example, two bonds to oxygen pull electron density away, making carbon more positive, while any bonds to hydrogen push electron density onto carbon, making it more negative. When both effects cancel out, the oxidation state of carbon in this molecule is 0.
You can apply this principle to determine the oxidation state of any molecule. Let’s take a more complex example—consider the blue carbon in an amide. That carbon is connected to oxygen, nitrogen, and another carbon. Since carbon-carbon bonds don’t count toward oxidation state calculations, we only consider the two bonds to oxygen and the bond to nitrogen. Since these three bonds pull electron density away from carbon, the oxidation state of this carbon is +3. Pretty simple!
Before we wrap up this quick tutorial, I want to remind you to be careful with implicit atoms. For instance, if we look at this green carbon over here, it is bonded to two other carbons and a nitrogen, but there is also an implicit hydrogen. If we forget to account for that hydrogen, we could miscalculate the oxidation state. Here, carbon-carbon bonds don’t count, one bond to nitrogen pulls electron density away, and the bond to hydrogen pushes electron density onto carbon. These effects cancel out, giving this carbon an oxidation state of 0.
As you can see, calculating oxidation states in organic molecules is incredibly easy. As long as you use this approach and know where elements are in the periodic table relative to carbon, you’ll always be able to determine oxidation states quickly and accurately.
