Mixed Aldol Condensation
Up to this point, we’ve only seen aldol condensations where two equivalents of the same molecule react with each other. For instance, in my previous tutorial, I demonstrated aldol condensation using acetone under both acidic and basic conditions, resulting in the corresponding α,β-unsaturated compound. Here’s another example where I take two equivalents of cyclopentanone, leading to an α,β-unsaturated compound with two rings. Or, consider a case where I use pentanone, forming a more complex-looking molecule. While I’m not showing the full mechanism for these reactions here, I challenge you to write it out based on what we discussed in the previous tutorials.
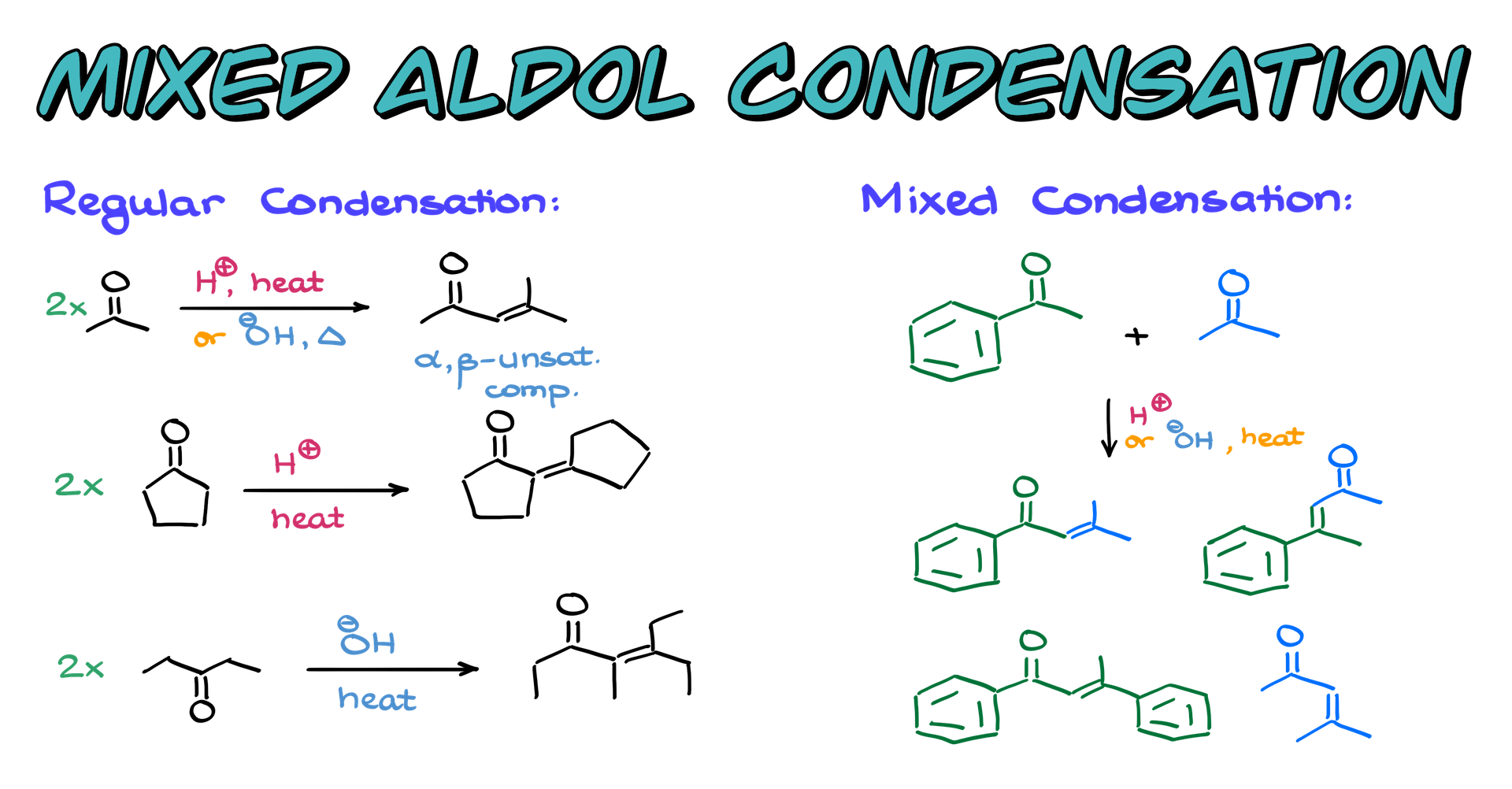
Now, the focus of this video is the mixed aldol condensation, where we react two different carbonyl compounds. To make it easier to follow, I’ll color-code my carbonyls: one green and one blue. If we mix these two molecules in either acidic or basic conditions and apply heat, we can end up with multiple possible products. We might get a combination where the green and blue molecules react in two different ways, forming two different α,β-unsaturated compounds. Alternatively, the green molecule could react with another green molecule, or the blue with another blue, leading to a mixture of products. This is the primary challenge of mixed aldol condensations—when we simply mix everything together, we get a messy “soup” of products, making it difficult to isolate the desired one.
So, how do we deal with this? There are actually three approaches.
1. Taking Advantage of Different Reactivities
The first strategy relies on the differing reactivity of our carbonyl compounds. Let’s look at a reaction between an aldehyde and a ketone under acidic conditions. Aldehydes are more polar and more electrophilic than ketones, meaning they are more likely to act as the electrophile in the reaction. The ketone, on the other hand, will generate a nucleophile via keto-enol tautomerism.
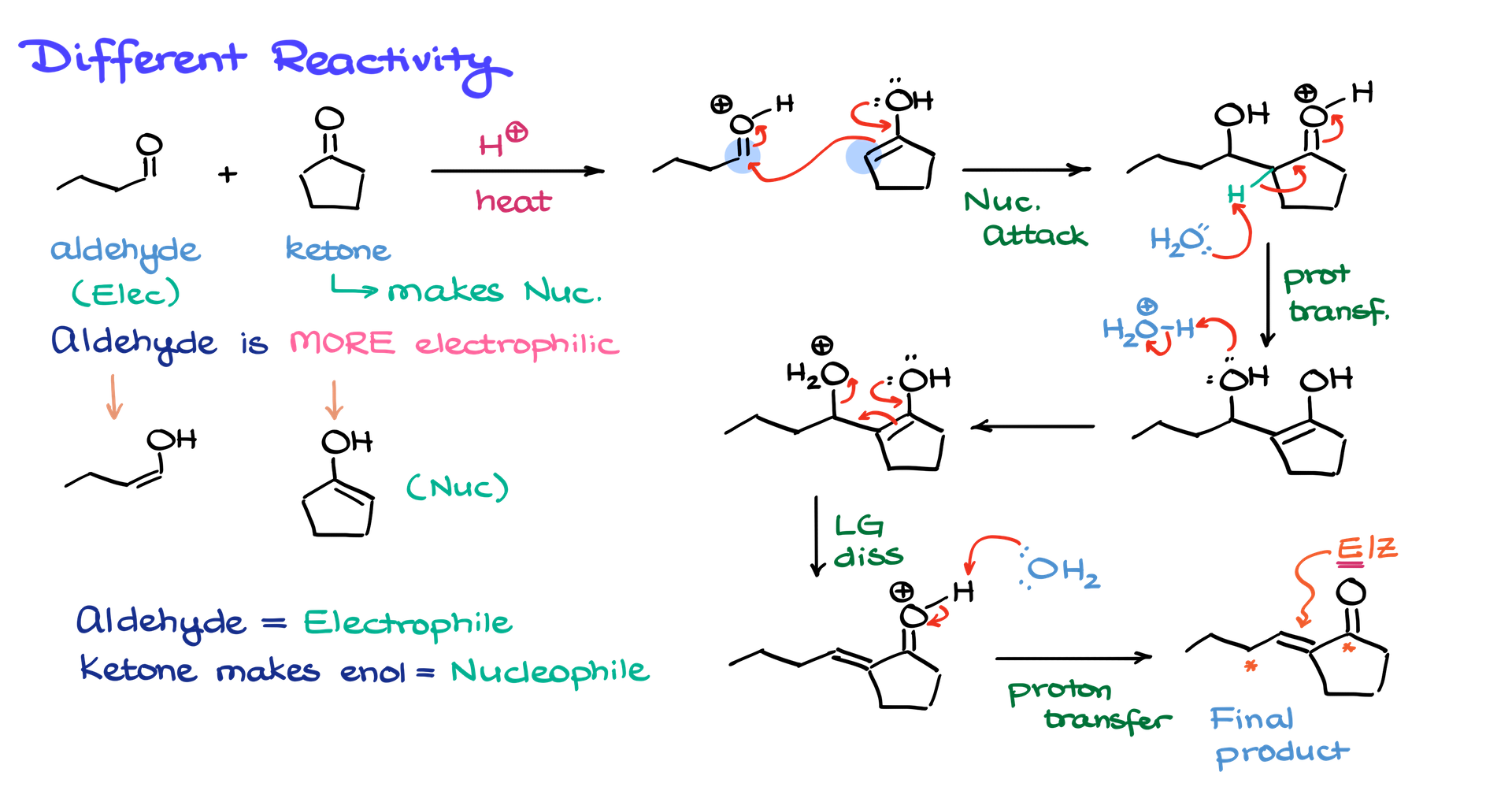
If we draw the possible enols that can form from both the aldehyde and the ketone, the aldehyde gives one type of enol, while the ketone forms another. Since keto-enol tautomerism in acidic conditions produces only small amounts of enol, the equilibrium mostly consists of the aldehyde and ketone in their standard forms. The aldehyde primarily acts as the electrophile, while the enolized form of the ketone serves as the nucleophile.
To illustrate the mechanism, we can protonate the aldehyde to enhance its electrophilicity. Then, the enol from the ketone will attack the aldehyde’s carbonyl carbon, forming a new C–C bond. This results in a protonated aldol intermediate. Because the reaction occurs at elevated temperatures, we won’t stop at the aldol stage; instead, we proceed to the elimination step, leading to the α,β-unsaturated product. This happens as we remove a proton adjacent to the carbonyl, form an enol, protonate the hydroxyl group to make it a good leaving group, and ultimately eliminate water, yielding the final product.
Although this method helps predict the major product, it is not entirely selective. Small amounts of enol can also form from the aldehyde, potentially leading to alternative products. However, the dominant outcome will involve the aldehyde acting as the electrophile and the ketone’s enol as the nucleophile.
2. Using a Non-Enolizable Carbonyl
A better approach involves reacting a non-enolizable carbonyl (one without α-hydrogens) with an aldehyde or ketone. For example, consider a reaction between benzaldehyde and butanal under acidic conditions at high temperature.
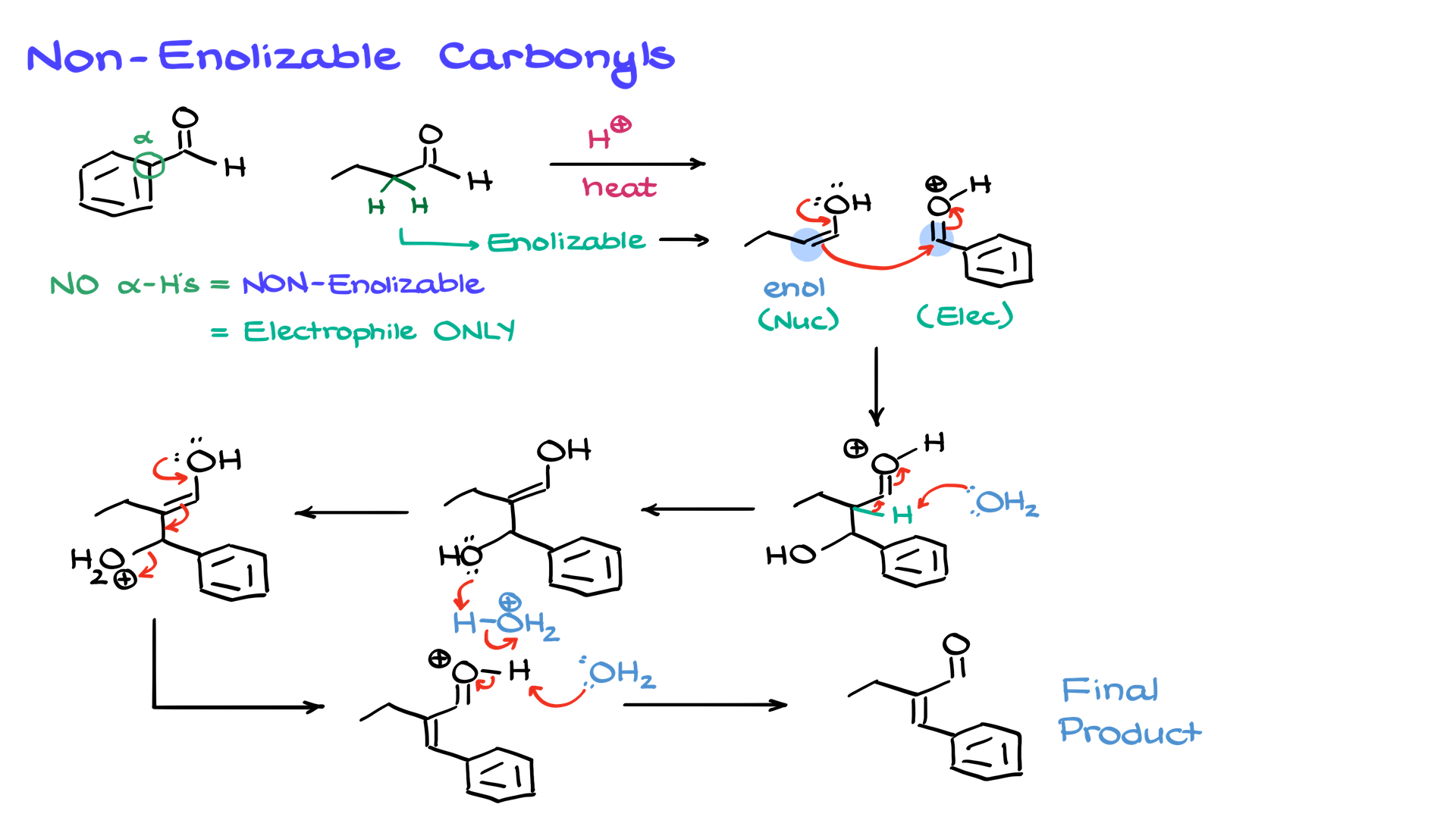
Both benzaldehyde and butanal are electrophilic, but benzaldehyde lacks α-hydrogens, meaning it cannot enolize. This means benzaldehyde can only act as an electrophile. Butanal, on the other hand, has α-hydrogens, allowing it to undergo keto-enol tautomerism and form an enol, which serves as the nucleophile.
The reaction proceeds similarly: protonating benzaldehyde enhances its electrophilicity, allowing the butanal enol to attack and form a new C–C bond. The aldol intermediate undergoes elimination under heat, producing an α,β-unsaturated product. While this method is more selective than the first, it still produces some side products since the butanal enol can react with itself as well.
3. Controlled Enolate Formation in Basic Conditions
The cleanest way to perform a mixed aldol condensation is to control enolate formation using strong bases in basic conditions.
Let’s revisit the original example where mixing two ketones led to a mess. Suppose our goal is to synthesize a specific product. To achieve this, we first determine which carbonyl will serve as the nucleophile and which as the electrophile. The α-carbon of the nucleophile must form a new bond with the carbonyl carbon of the electrophile.
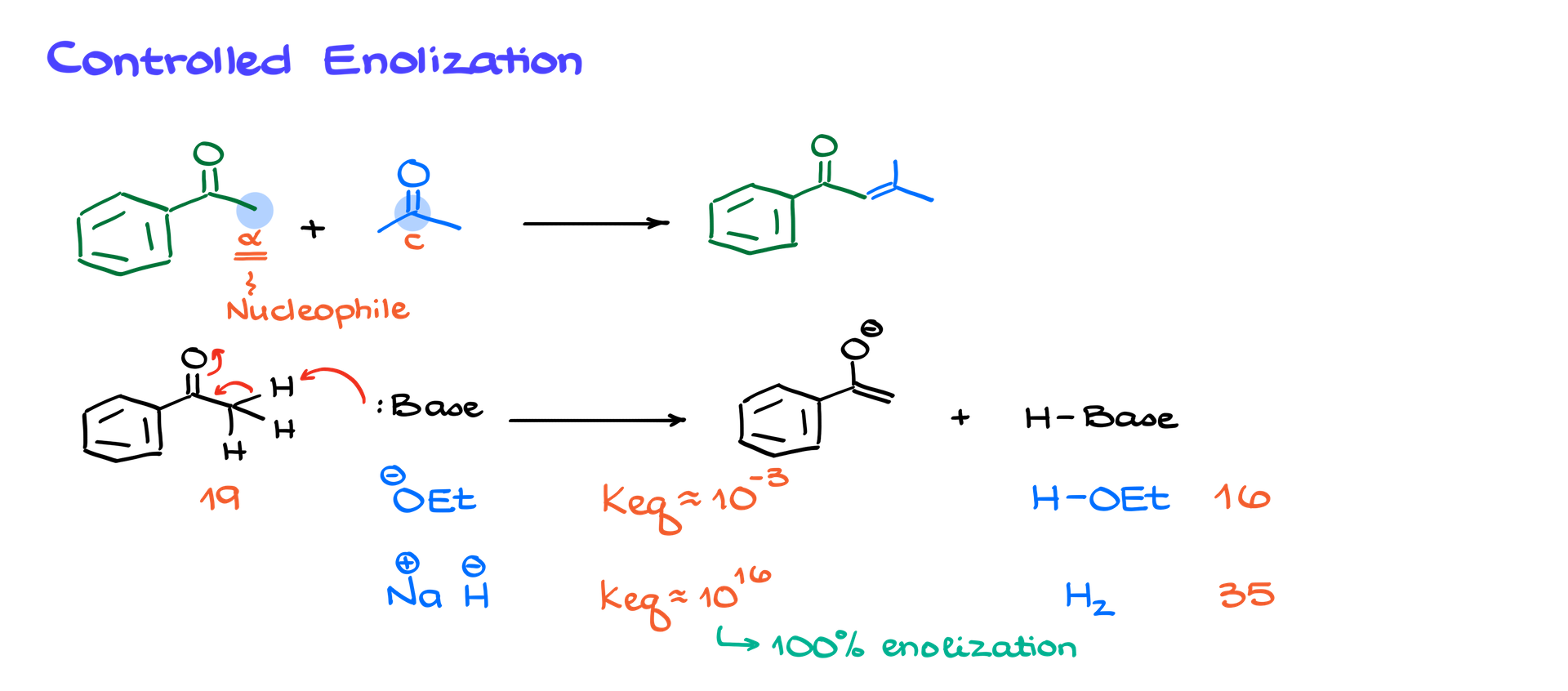
To ensure selectivity, we first convert one ketone (let’s say acetophenone) into an enolate using a strong base like sodium hydride (NaH). This step is crucial because the choice of base determines whether enolization is complete. Using a weak base like ethoxide (EtO⁻) results in only partial enolate formation (~1%), whereas a strong base like sodium hydride leads to nearly 100% enolate formation.
Once we have a fully enolized acetophenone, we introduce the second carbonyl compound. The enolate attacks the electrophile’s carbonyl carbon, forming a new C–C bond. A simple acidic workup yields the aldol product, while heating induces elimination to give the α,β-unsaturated compound. This stepwise approach allows for high selectivity and avoids side reactions.
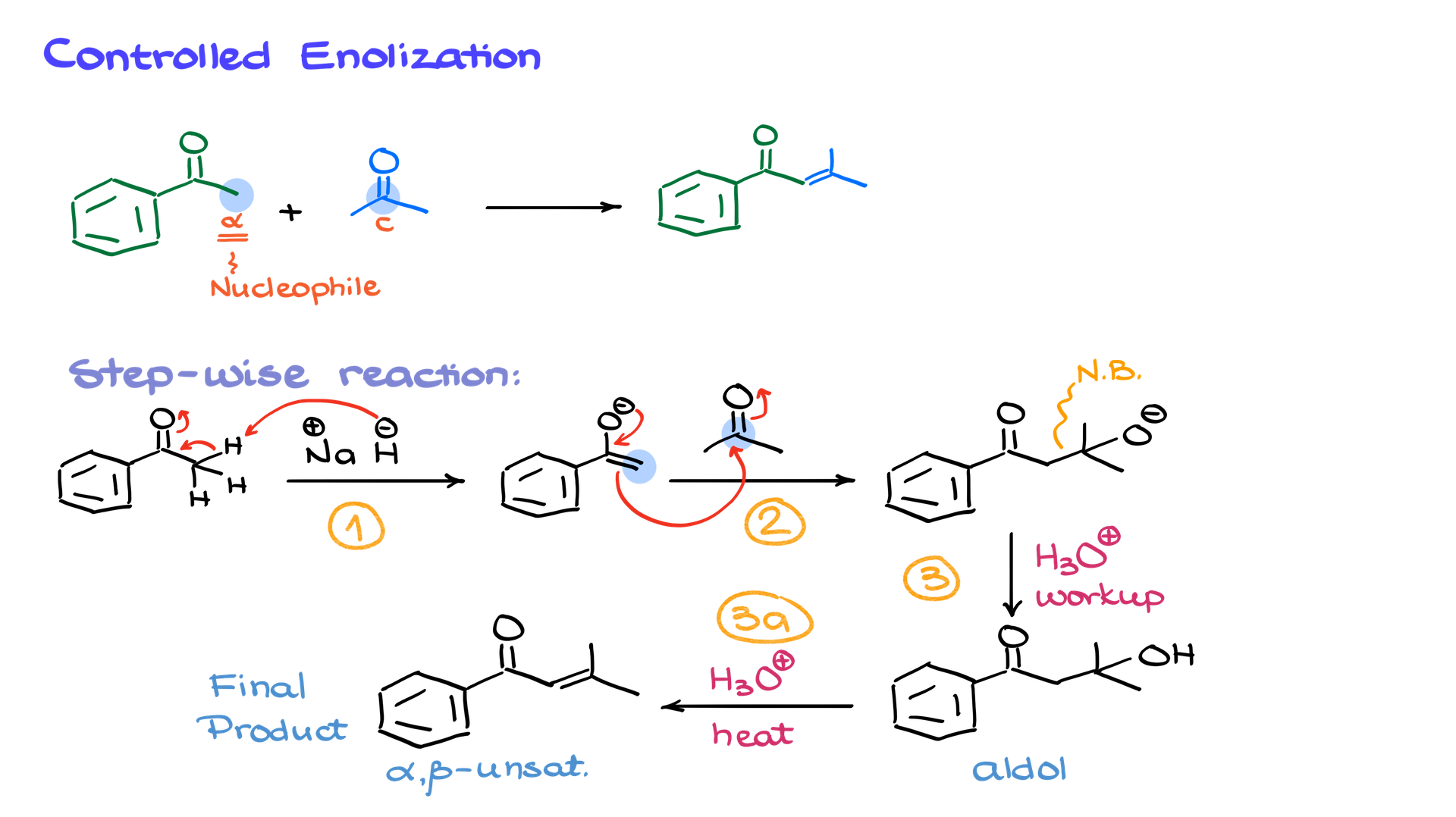
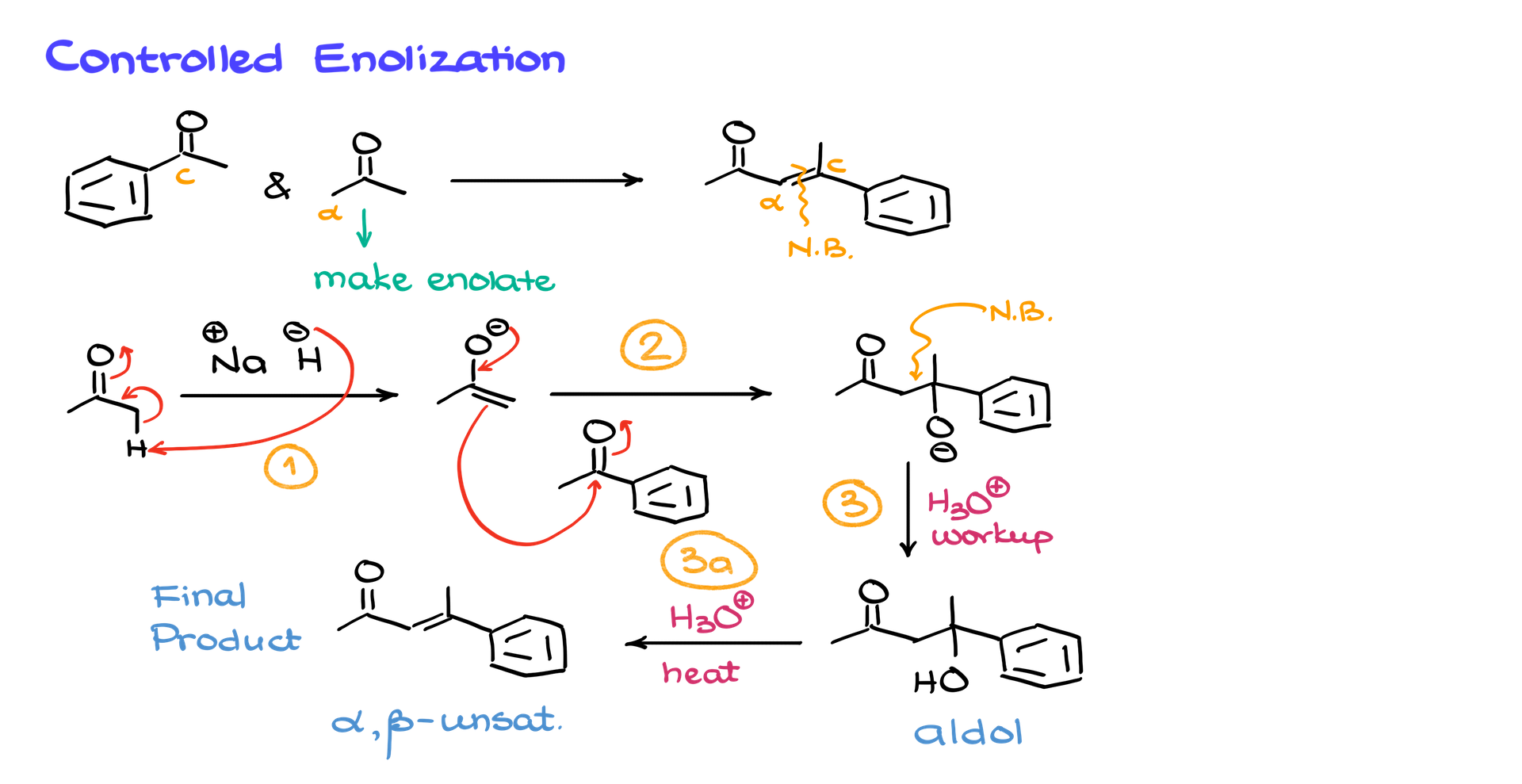
If we wanted to make a different product—for example, forming a double bond at another location—we would enolize a different starting material. Suppose we now want to enolize acetone instead of acetophenone. We would treat acetone with a strong base, forming its enolate, then add acetophenone as the electrophile. The reaction follows the same steps, ultimately leading to a different α,β-unsaturated product.
Key Takeaways
- If aldehydes and ketones are mixed, the aldehyde will typically act as the electrophile, and the enolized ketone will be the nucleophile. However, side reactions are inevitable.
- Using a non-enolizable carbonyl prevents unwanted enolate formation. This method reduces the number of possible products but does not eliminate them entirely.
- The best approach for clean selectivity is controlled enolate formation in basic conditions. By fully enolizing one carbonyl before adding the second, we ensure that only the desired reaction occurs.
If you want a clean mixed aldol condensation, always aim for controlled enolate formation. First, selectively enolize the desired carbonyl using a strong base. Then, introduce the electrophile to form a new C–C bond. Finally, perform a workup to obtain either the aldol product or the α,β-unsaturated compound via elimination.
This method ensures precise control over the reaction, minimizing side products and maximizing the yield of the desired product.
