Reaction of Carboxylic Acids with Organometallic Reagents
In this tutorial, I want to talk about how carboxylic acids react with organometallic compounds, such as Grignard reagents and organolithium compounds.
Reaction with Grignard Reagents
First, let’s take a look at the reaction between carboxylic acids and a Grignard reagent. For my carboxylic acid, I’ll use butanoic acid, and for simplicity’s sake, I’ll use methyl magnesium bromide as the Grignard reagent.
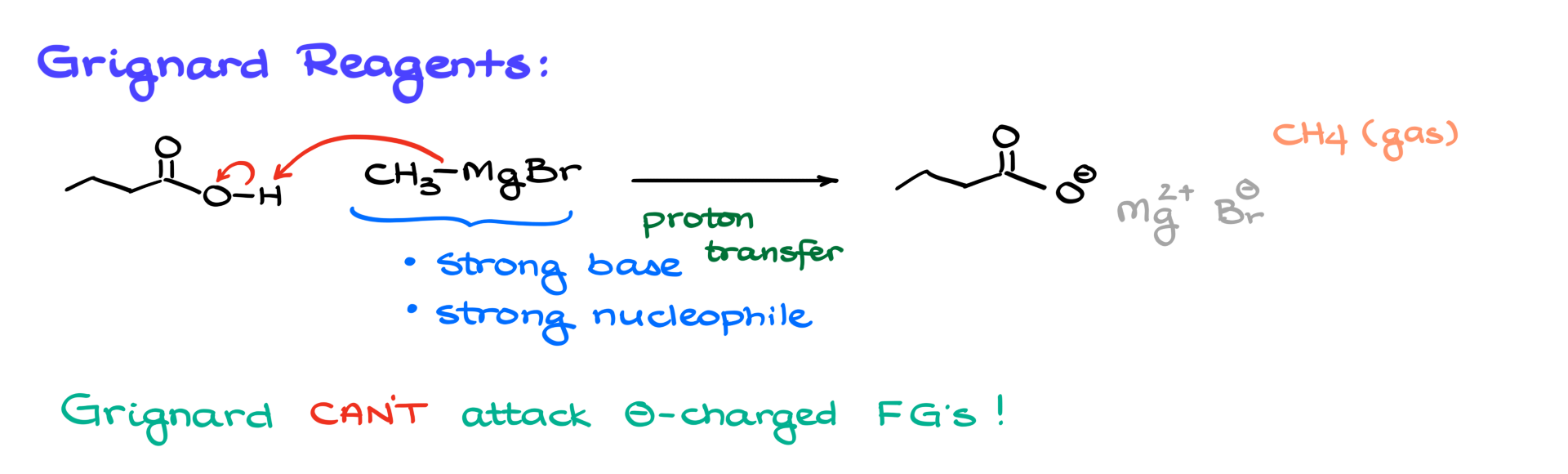
When it comes to organometallics like Grignard reagents, we know that these molecules are strong bases and also strong nucleophiles. So, if we bring a Grignard reagent into contact with anything acidic—like a carboxylic acid, for instance—we naturally expect an acid-base reaction. In this case, our methyl magnesium bromide will snatch the proton from the carboxylic acid, forming the corresponding carboxylate. The magnesium will act as the counterion, and our conjugate acid, in this case, will be methane, which, being a gas, will simply escape. Since Grignard reagents are not nucleophilic enough to attack negatively charged functional groups, that’s the end of the story. The Grignard reagent will only undergo the acid-base reaction with carboxylic acids—nothing more.
Reaction with Organolithium Compounds
Now, when it comes to organolithium compounds, things get a little more interesting. Let’s take the same butanoic acid as our starting material, and like before, I’ll use a simple organolithium compound—methyl lithium. Just like Grignard reagents, organolithium compounds are also very strong bases and strong nucleophiles. So, the first step will again be an acid-base reaction, where methyl lithium deprotonates the carboxylic acid. This gives us the carboxylate, but this time with lithium as the counterion instead of magnesium. And, just like before, methane gas will be released.
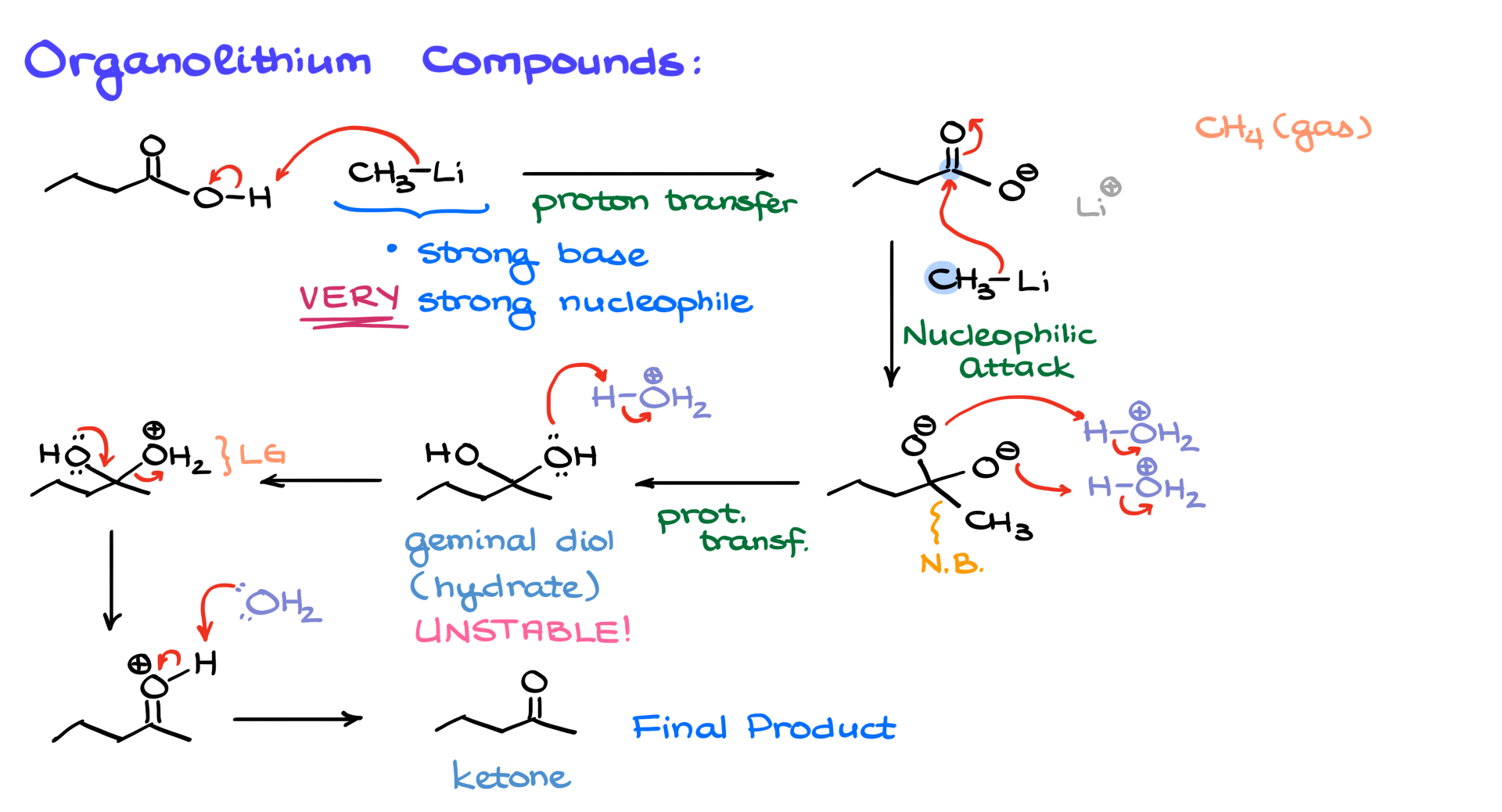
But here’s where the big difference lies between organolithium compounds and Grignard reagents. When I said that organolithium compounds are strong nucleophiles, I may have understated just how strong they are. I should have said they are very strong nucleophiles—they don’t care whether the functional group is negatively charged or not; they will still attack. So, if we have a second equivalent of methyl lithium (and these reactions are typically done in excess of our organometallic reagent), this second equivalent will attack the carbonyl carbon, forming a new carbon-carbon bond between the carbonyl and the methyl group from the organolithium compound. As a result of this nucleophilic attack, we end up with a species containing two negatively charged oxygens on the same carbon—a geminal dianion.
This geminal dianion is a highly unstable and extremely reactive species. Even strong nucleophiles like organolithium compounds can’t push the reaction any further at this point. This is where the reaction stops, and we proceed with an acidic workup. Adding acid will protonate both negatively charged oxygens, forming a geminal diol—also known as a hydrate. However, geminal diols are generally unstable and tend to collapse into their corresponding carbonyl compounds.
Since we are still in acidic conditions, one of the hydroxyl groups gets protonated, forming water—a good leaving group. This allows for an assisted ionization, where the oxygen helps expel water, generating a protonated carbonyl intermediate. This intermediate is then deprotonated, yielding a ketone as the final product of the reaction.
Now, you might be wondering whether this ketone can react further with the organolithium compound we started with. The answer is no. By the time we reach the ketone, the organolithium compound is no longer present because it was consumed in the earlier steps. After forming the geminal dianion, we carried out an acidic workup, which would have destroyed any remaining methyl lithium. This makes the reaction an excellent method for converting carboxylic acids into ketones using just an organolithium reagent.
While you might think this reaction is wasteful—since it requires two equivalents of the organolithium compound, one for deprotonation and one for nucleophilic attack—the simplicity of the procedure outweighs economic concerns. Many simple organolithium reagents, like methyl lithium and butyl lithium, are commercially available and relatively inexpensive. So, the next time you see a carboxylic acid reacting with an organometallic compound, remember: if it’s a Grignard reagent, the reaction stops at acid-base chemistry, but if it’s an organolithium compound, you’ll end up with a ketone.
