Alkylation of Enolates
The alpha-alkylation of carbonyl compounds is a foundational reaction in organic synthesis, enabling the formation of new carbon-carbon bonds with precision. This reaction is not only a common topic in the second semester organic chemistry curriculum but also widely used in practical applications to construct complex molecular frameworks. In this tutorial, we’ll break down the mechanism, explore its synthetic versatility, and discuss key considerations to maximize efficiency and selectivity.
Introduction to the Reaction
Let’s start by looking at the general scheme of the reaction. In a nutshell, we take a carbonyl compound (e.g., acetone as our example) and treat it with a strong base to form an enolate anion. This enolate, acting as a nucleophile, reacts with an alkyl halide to create a new carbon-carbon bond.
Key Points to Consider
Before jumping into the mechanism, here are a few critical points you need to know:
1. Complete Enolization is Required:
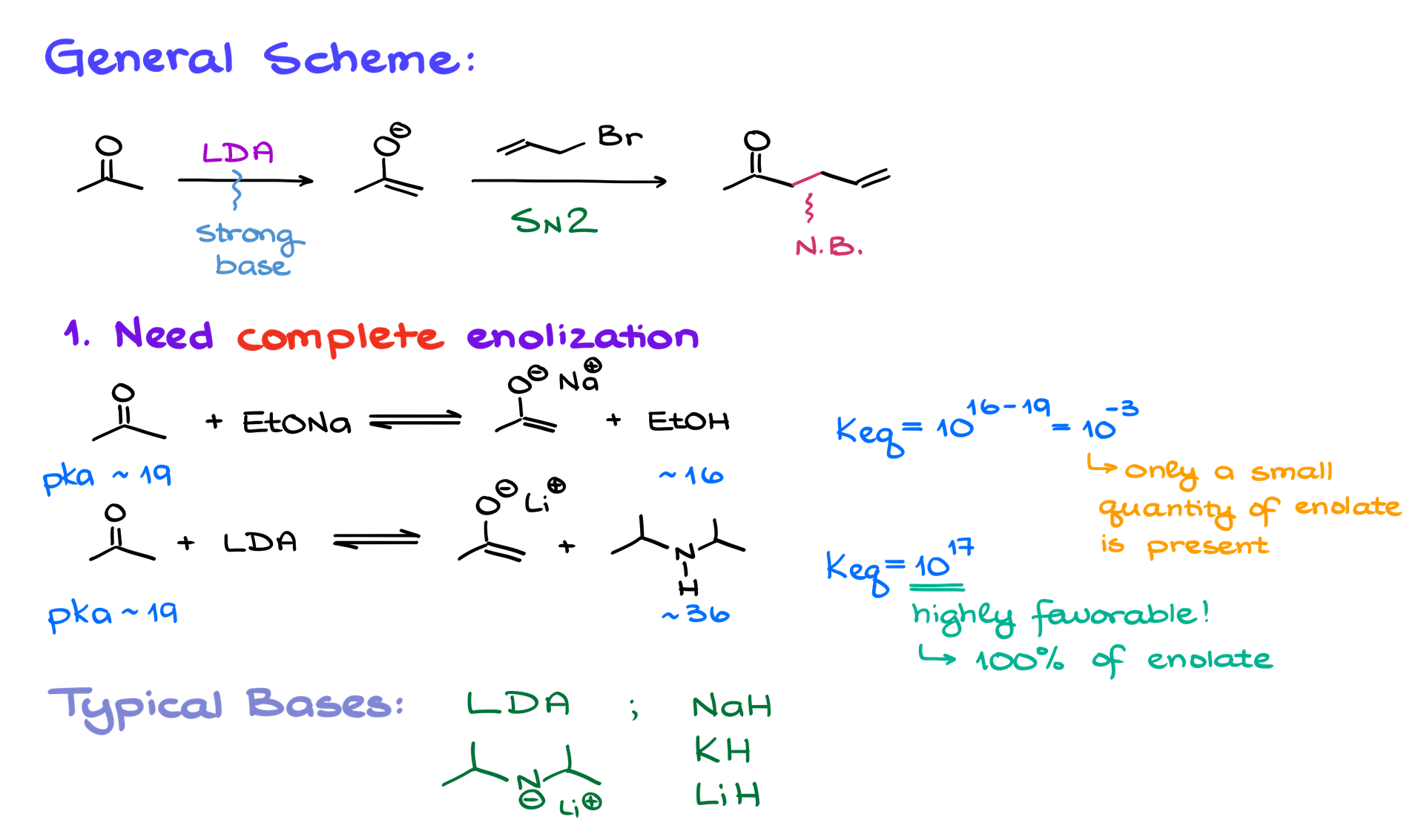
The first step of this reaction requires a strong base to fully enolize the carbonyl compound. Using a weaker base (e.g., sodium ethoxide) will only result in partial enolization, as the equilibrium constant heavily favors the starting material due to pKa differences (acetone ~19, ethanol ~16). This low enolate concentration leads to side reactions, primarily between the base and the alkyl halide, resulting in low yields. You might wanna review the enolization of carbonyls and the keto-enol tautomerism concepts as we’re going to be using them a lot throughout this tutorial.
However, using a strong base like lithium diisopropylamide (LDA) shifts the equilibrium completely toward enolate formation. For example, the equilibrium constant in this case is highly favorable, around 1017, ensuring nearly 100% enolate formation and minimizing side reactions.
2. Enolate vs. Enol:
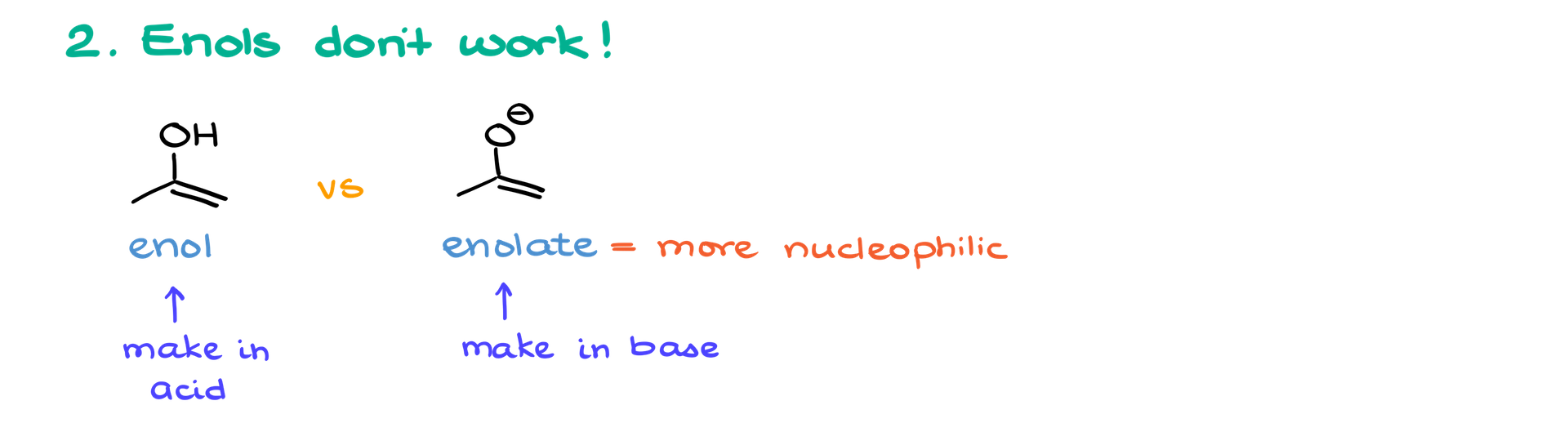
Enolates are significantly more nucleophilic than enols due to their negative charge. As a result, enolates are typically used in these reactions, whereas enols (formed under acidic conditions) are ineffective. Since this reaction is performed under basic conditions, enol formation is avoided altogether.
Suitable Carbonyl Compounds for Alkylation
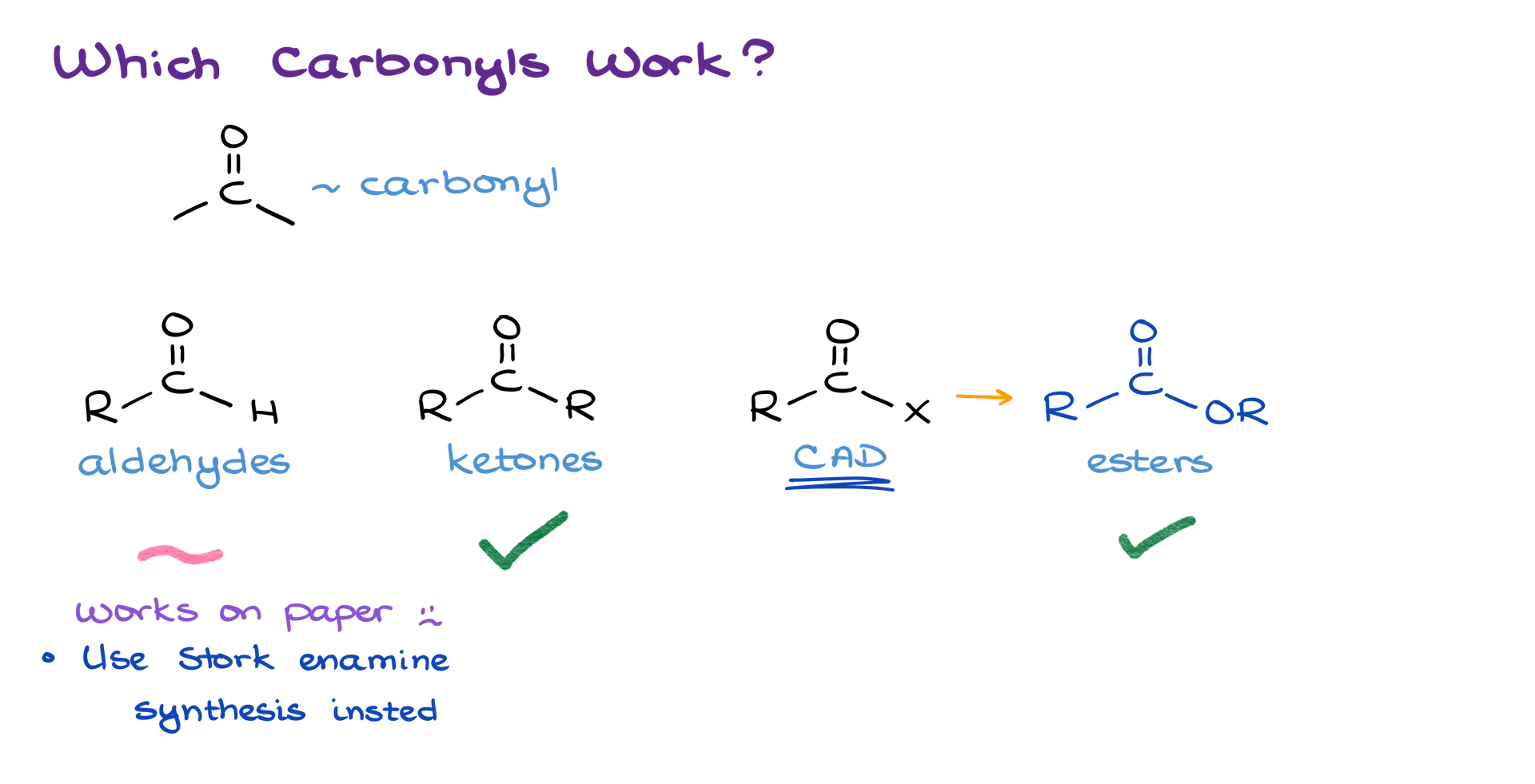
Among the large family of carbonyl-containing compounds, only certain types are suitable for alpha-alkylation:
- Ketones: The most common participants, frequently used in these reactions.
- Esters: Usable but less common due to their lower reactivity.
- Aldehydes: These are challenging to enolize with strong bases. For practical purposes, alpha-alkylation of aldehydes is better achieved via the Stork enamine synthesis, which creates enamine intermediates. While some textbooks and instructors may present direct enolization of aldehydes, this often only works on paper.
• Other Derivatives: Amides and acid chlorides, while theoretically possible, present numerous challenges and are rarely employed for this purpose.
Mechanistic Overview
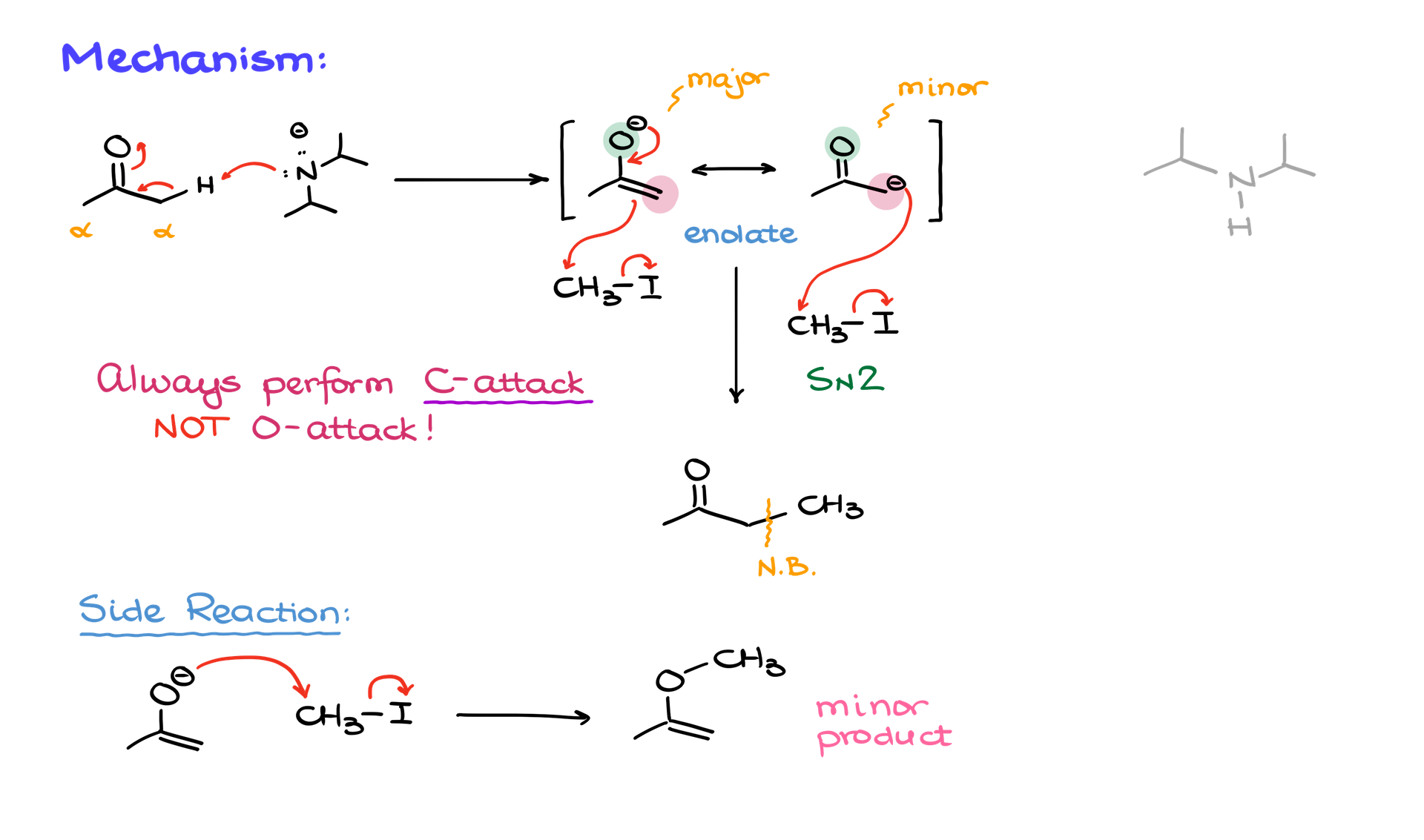
The alkylation reaction proceeds in two key steps:
1. Enolization:
Using a strong base like LDA or sodium hydride, the alpha hydrogen of a carbonyl compound is deprotonated to form a resonance-stabilized enolate. This enolate can be represented in two forms:
• The major resonance contributor (negative charge on oxygen).
• The minor resonance contributor (negative charge on carbon).
2. SN2 Reaction with Alkyl Halide:
The enolate’s carbon (not oxygen) performs a nucleophilic attack on the electrophilic carbon of an alkyl halide, displacing the halide ion. This step is sensitive to steric effects, favoring primary alkyl halides, allylic halides, or benzylic halides. Secondary halides react sluggishly, and tertiary halides are typically unreactive.
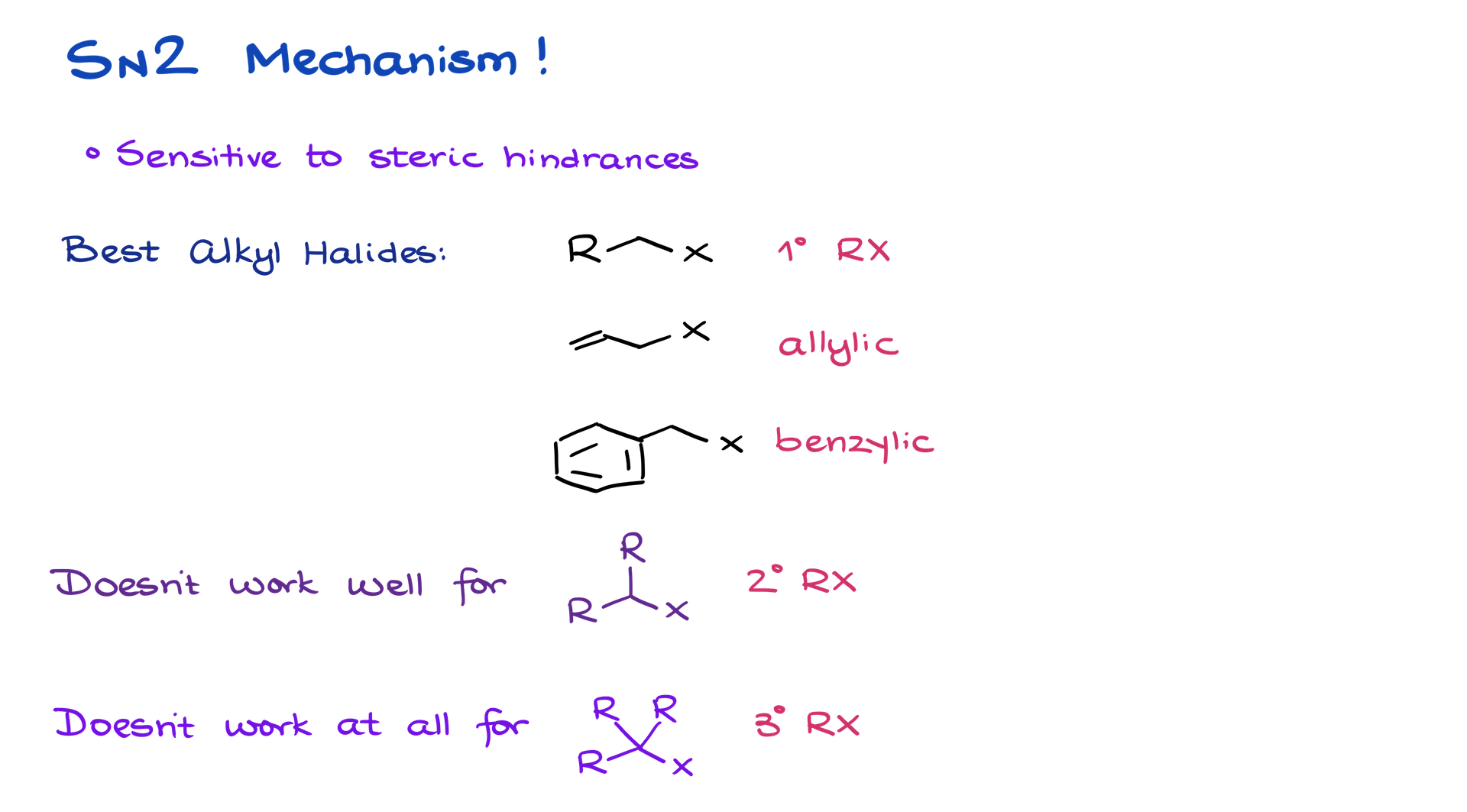
The product is a new carbon-carbon bond, with the reaction favoring carbon nucleophilicity over oxygen due to conditions that suppress side reactions.
Synthetic Applications and Examples
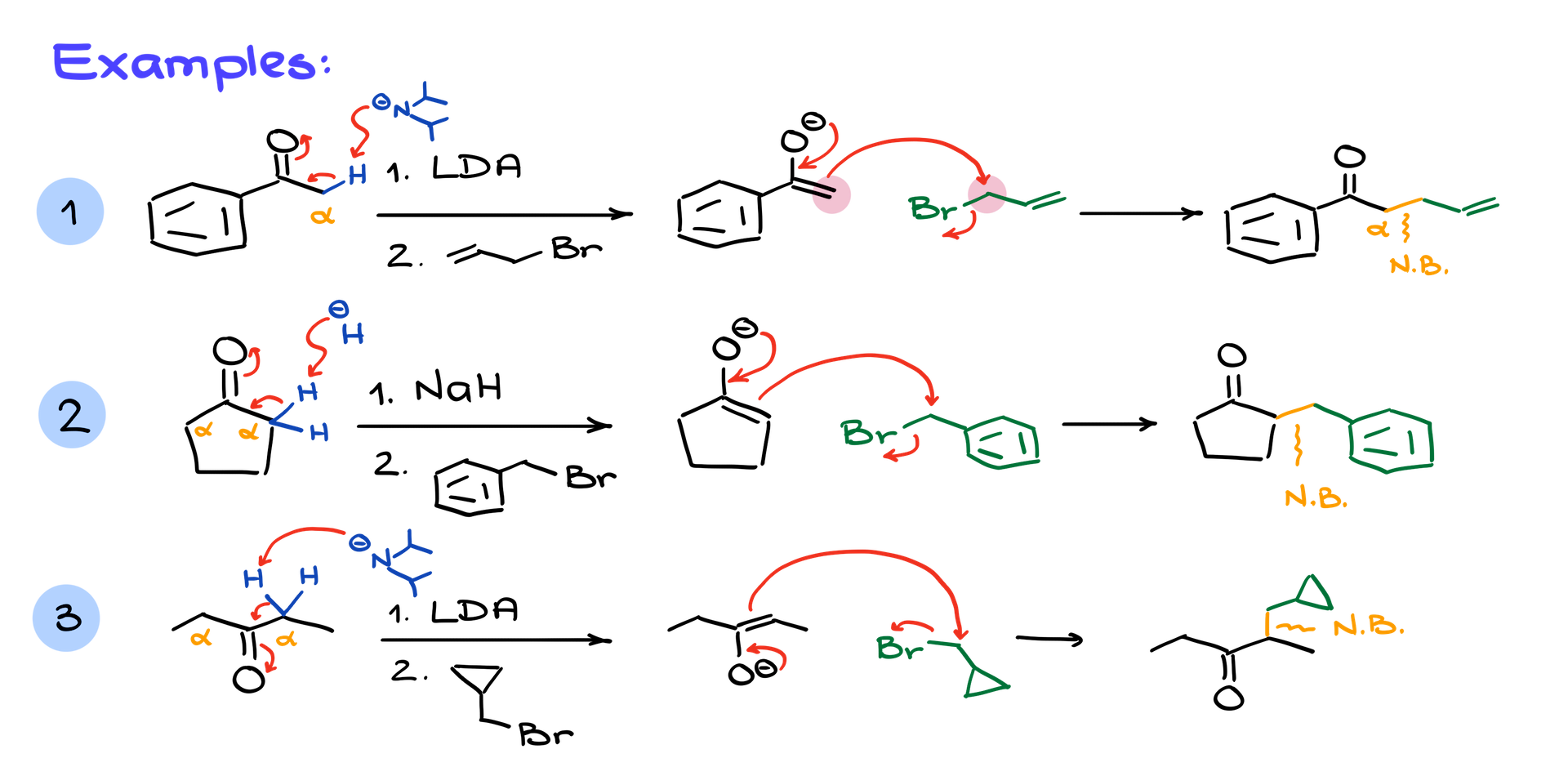
1. Acetophenone with Allyl Bromide:
Treating acetophenone with LDA forms the enolate at the single alpha position. The enolate then reacts with allyl bromide in an SN2 mechanism to form the product with a new C-C bond.
2. Cyclopentanone with Benzyl Bromide:
Symmetrical cyclopentanone is enolized by sodium hydride, with either alpha position leading to the same enolate. This enolate reacts with benzyl bromide, forming a benzyl-substituted cyclopentanone.
3. 3-Pentanone with a Complex Halide:
Using LDA, the symmetrical 3-pentanone forms an enolate at either alpha position. Reacting this enolate with a branched alkyl halide yields a unique product with an extended carbon chain.
4. Unsymmetrical Ketones:
One of the most powerful aspects of this reaction is the ability to selectively form new carbon-carbon bonds. When working with unsymmetrical carbonyl compounds, the choice of base and conditions allows selective enolization:
• Thermodynamic Enolate Formation: Using slower-acting base like sodium hydride or sub-stoichiometric LDA favors the more substituted, stable enolate.
• Kinetic Enolate Formation: Using excess fast-acting LDA favors the less substituted, kinetically favored enolate.
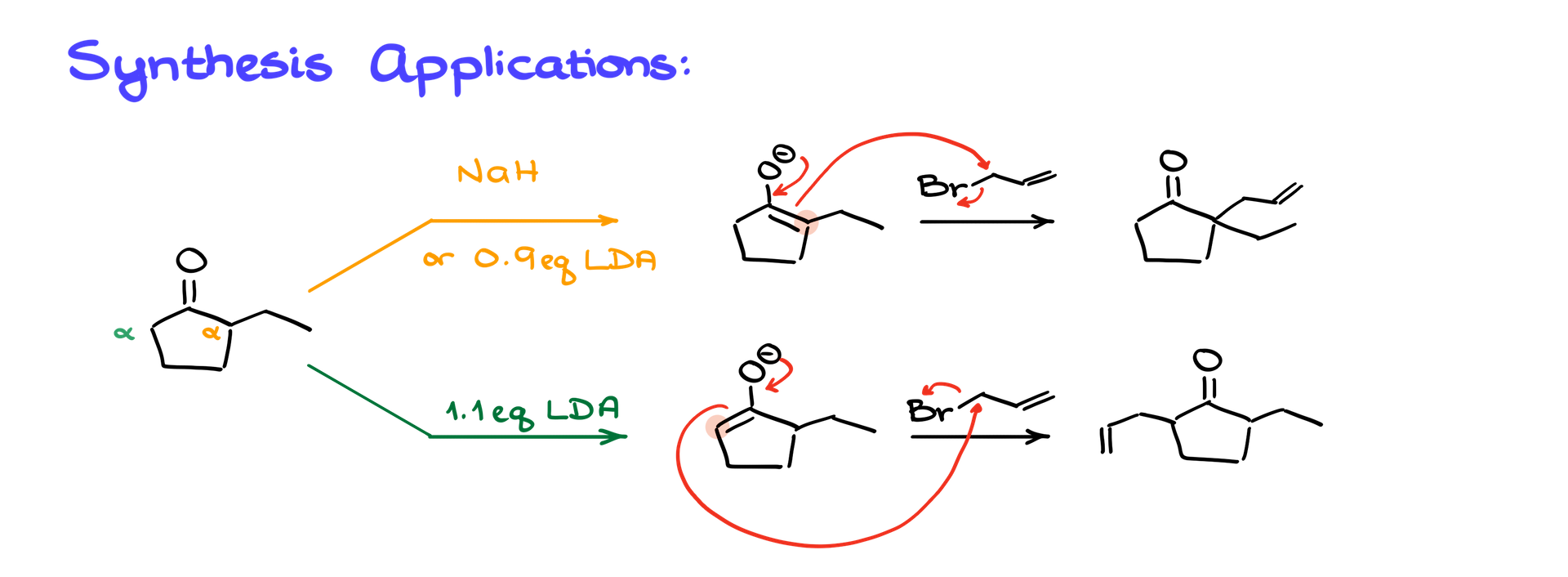
This selective enolate formation enables targeted synthesis of different products depending on the site of alkylation.
