Axial Leaving Group Reactivity
In this tutorial, we’ll explore how to analyze chair conformations to predict the outcomes of common organic reactions, such as SN2 and E2. Using a typical exam question as our starting point, we’ll examine how the positioning of substituents in six-membered rings—specifically in axial or equatorial positions—impacts the reactivity of leaving groups and the accessibility of reaction pathways. Along the way, we’ll address common misconceptions about the relationship between stability and reactivity and highlight the importance of steric and stereoelectronic factors in determining reaction rates and outcomes.
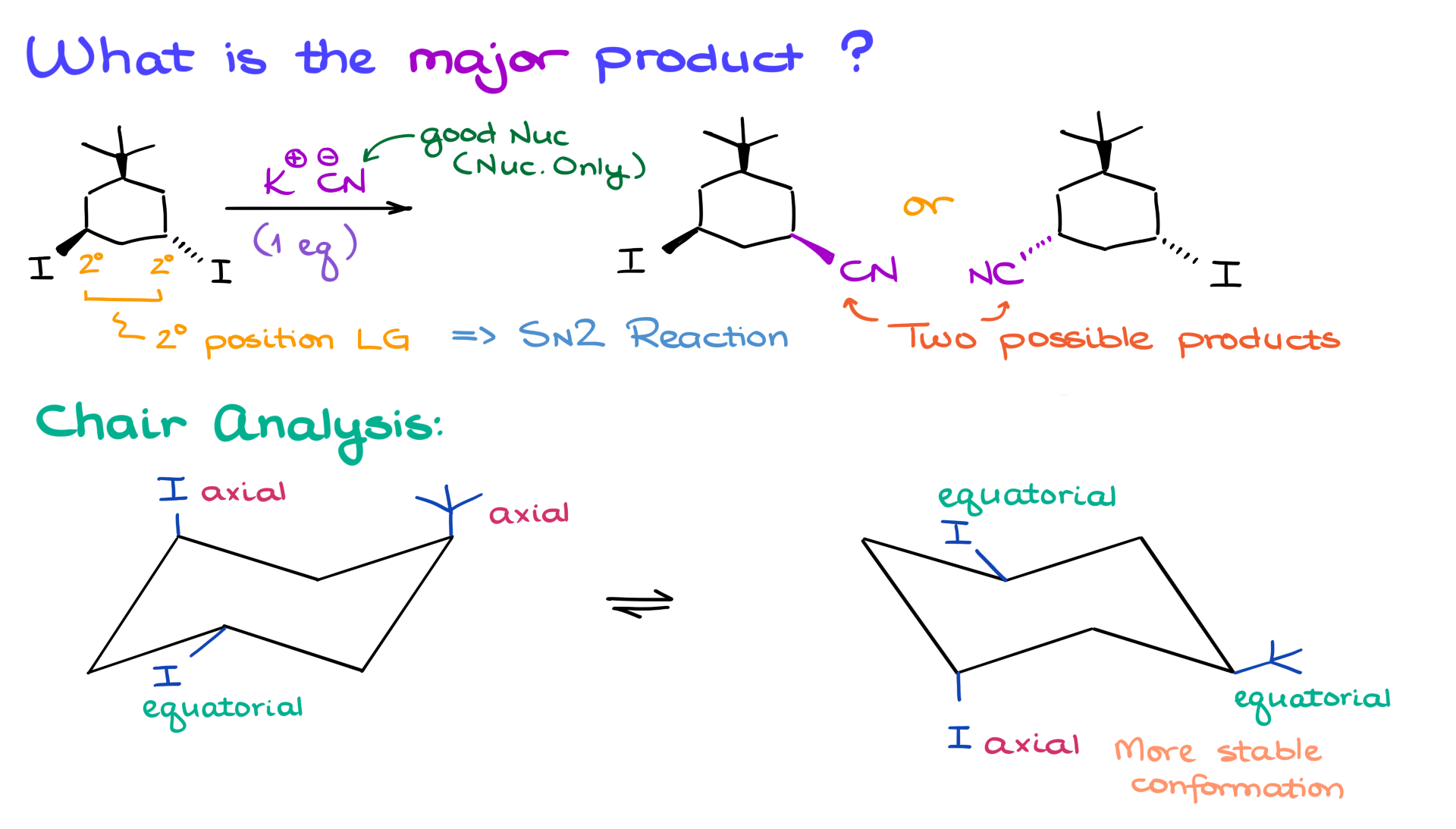
We’re working with potassium cyanide, a strong nucleophile. The reaction involves a six-membered ring with two leaving groups, both in secondary positions. This setup suggests an SN2 reaction, which means we have two possible products.
But here’s the key question: Which of these two products will be the major product?
Analyzing Chair Conformations
Since we’re dealing with a six-membered ring, we need to examine the reaction using chair conformations. Let’s begin by drawing the two possible chair forms, including their flipped versions, and add the substituents.
If you need help with drawing chair conformations, I’ve created a detailed tutorial on that—check it out for a refresher. Back to our example: in one chair conformation, both groups are in axial positions; in the other, both are in equatorial positions.
Now, it’s critical to note that the chair conformation on the right is more stable because the bulky tert-butyl group is in the equatorial position.
Relating Chair Conformations to SN2 Reactivity
How does this help us determine the major product? Let’s quickly review the mechanics of an SN2 reaction.
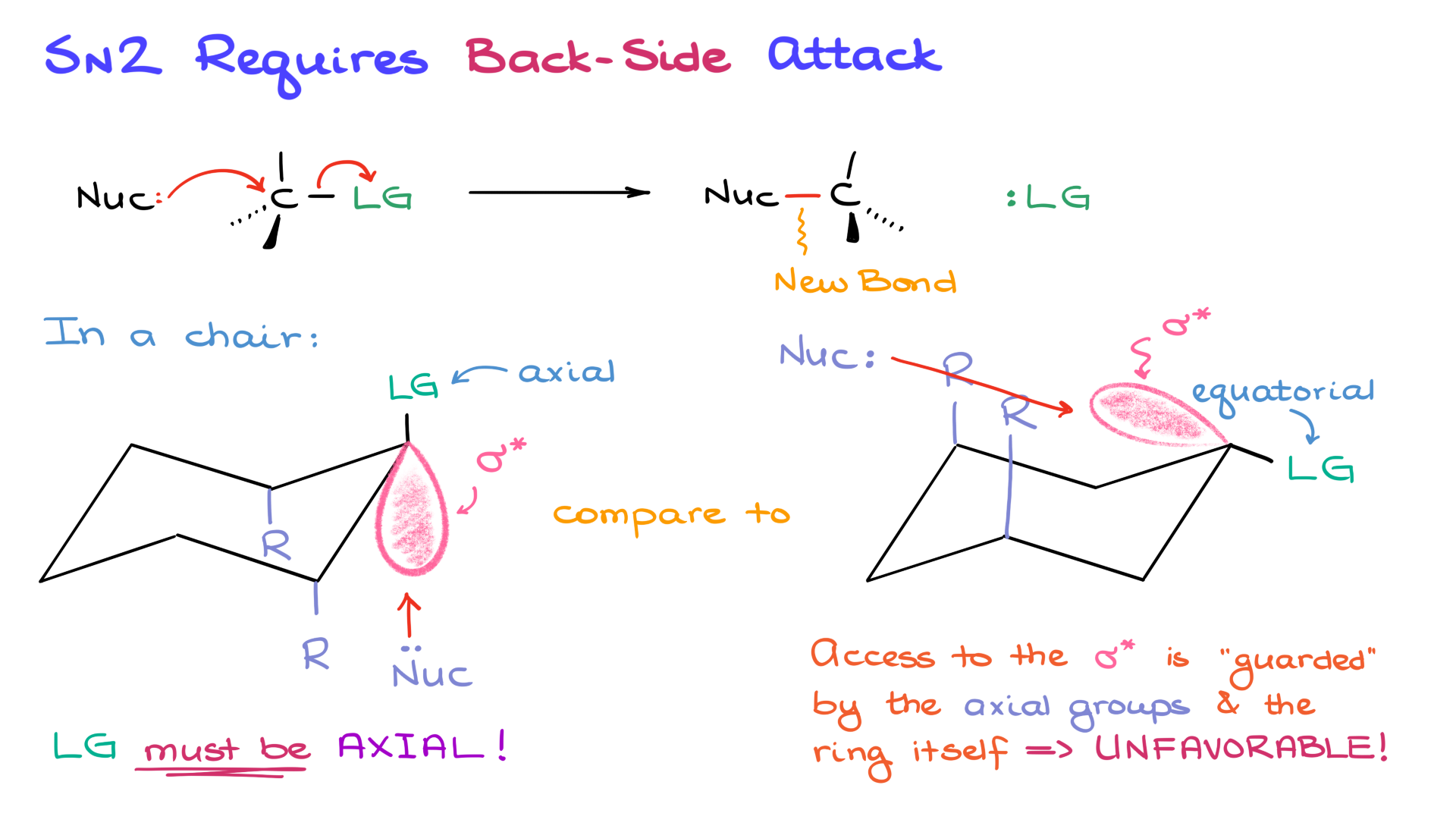
SN2 reactions require a backside attack, where the nucleophile approaches the carbon atom opposite the leaving group. The angle of attack must be 180° to the bond with the leaving group. This alignment is necessary to supply electron density to the anti-bonding sigma star orbital and displace the leaving group.
Let’s apply this to our chair conformations:
1. Axial leaving group: The anti-bonding orbital aligns well for a backside attack, with minimal steric hindrance. The nucleophile has clear access.
2. Equatorial leaving group: The anti-bonding orbital is obstructed by axial groups, creating steric interference. This makes the attack unfavorable.
Thus, the leaving group must be in the axial position for a successful backside attack.
Determining the Major Product
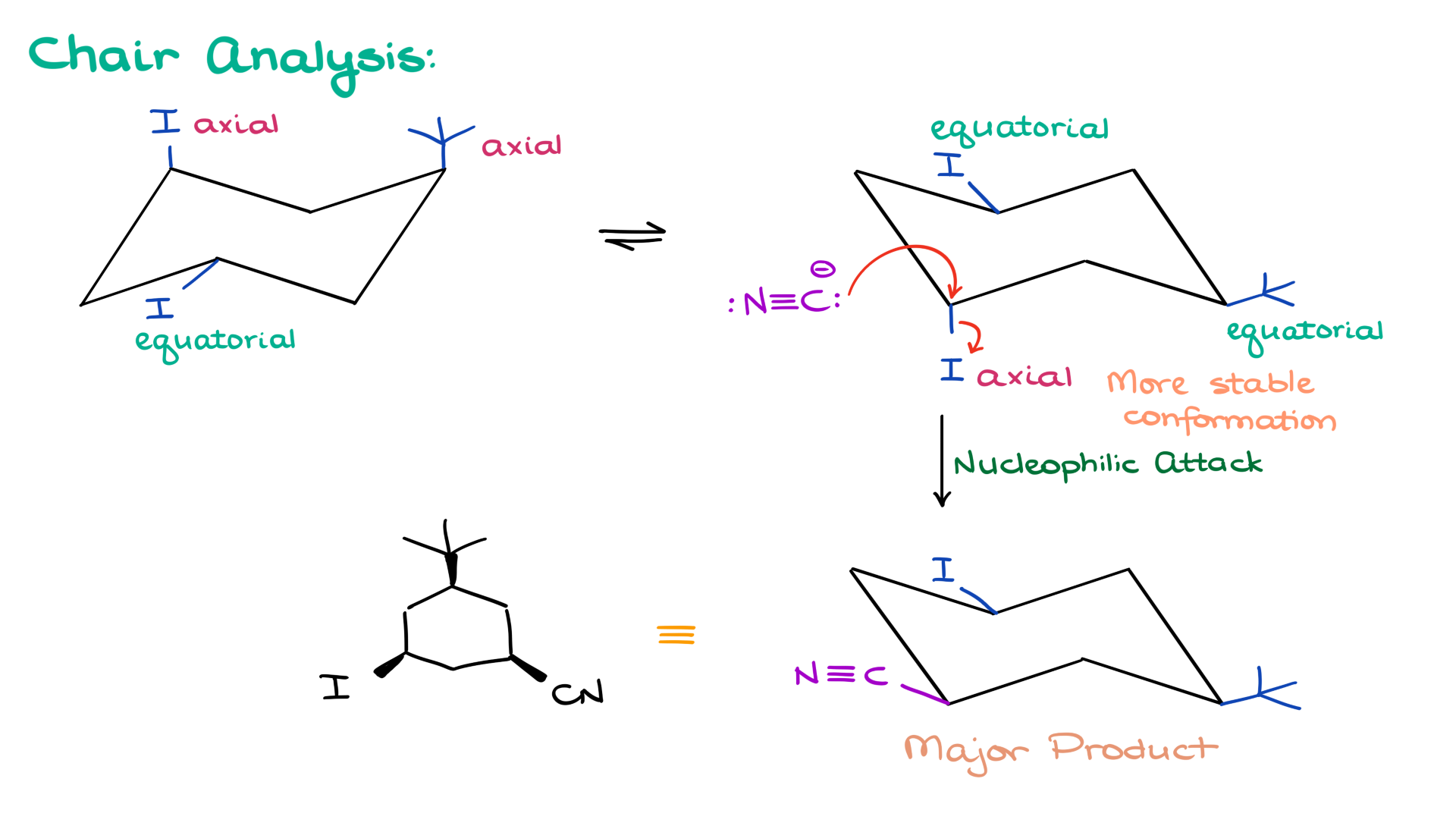
Returning to our original example: while the conformation with equatorial groups is more stable, the reaction requires the leaving group to be axial. In the more stable chair conformation, iodine occupies the axial position, making it the reactive site. The nucleophile attacks this iodine, resulting in the major product. The final product can be redrawn with dashes and wedges, showing the substitution clearly.
Comparing Two SN2 Reactions
Next, let’s compare two seemingly similar SN2 reactions, both involving sodium azide as the nucleophile. Reaction A has the leaving group pointing away, while Reaction B has it pointing towards us.
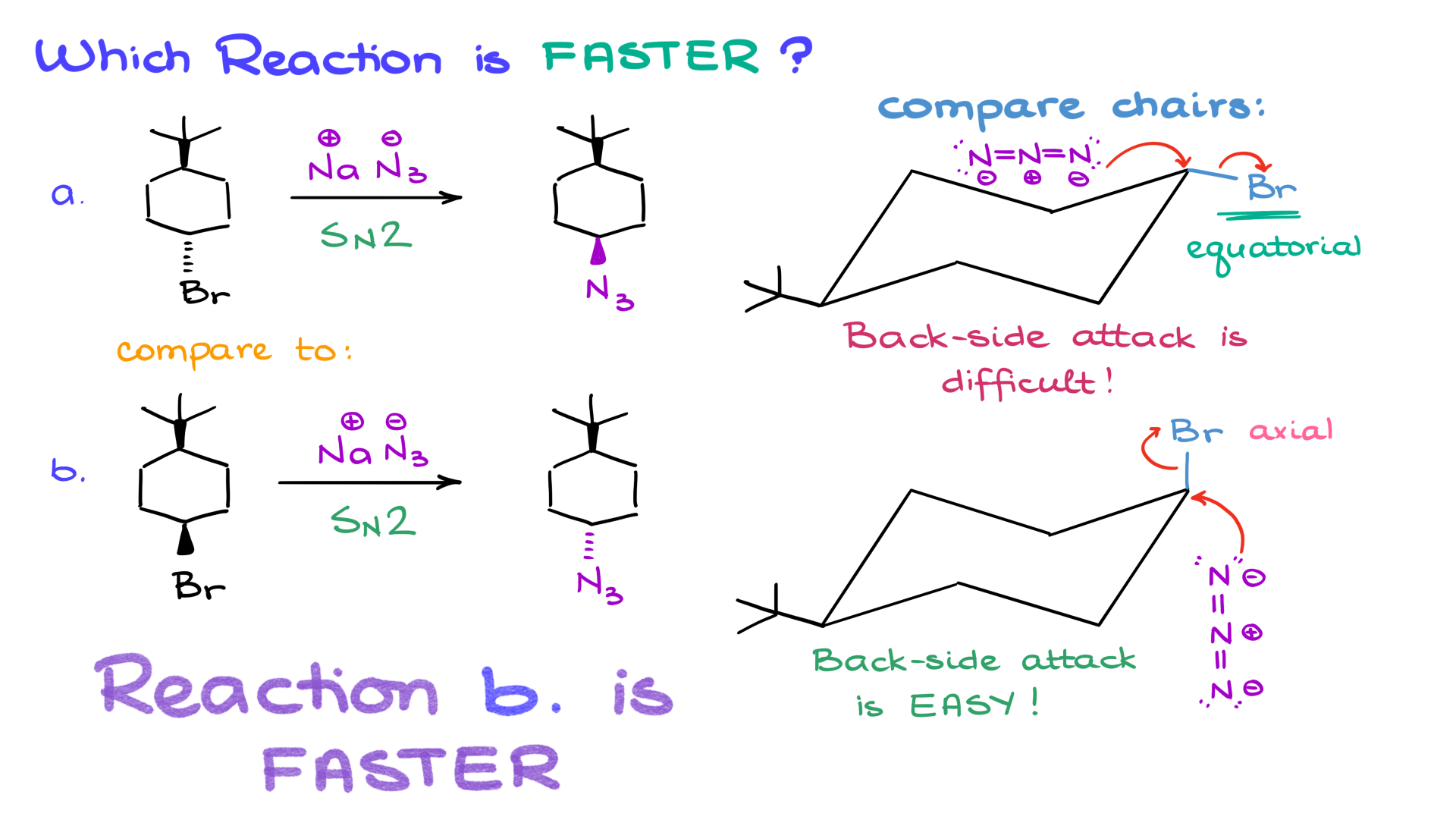
1. Molecule A: The most stable chair conformation shows the leaving group in the equatorial position. Backside attack is difficult due to steric hindrance.
2. Molecule B: The leaving group is in the axial position. Here, backside attack is straightforward, allowing the reaction to proceed faster.
This example underscores a key point: reaction rate depends on more than stability. While the starting material in Reaction B is less stable, it’s more reactive because the leaving group is in the ideal axial position.
Misconceptions About Stability and Reactivity
A common misconception in organic chemistry is that stability correlates directly with reactivity. This isn’t always true. For reactions like SN2, steric and stereoelectronic factors play a more significant role. In Reaction B, despite its lower stability, the axial positioning of the leaving group makes it more reactive.
Applying Chair Conformations to E2 Reactions
Let’s shift to E2 reactions in chair conformations. Consider two reactions that yield the same alkene product. Reaction A has the leaving group pointing in one direction, while Reaction B has it pointing the opposite way.
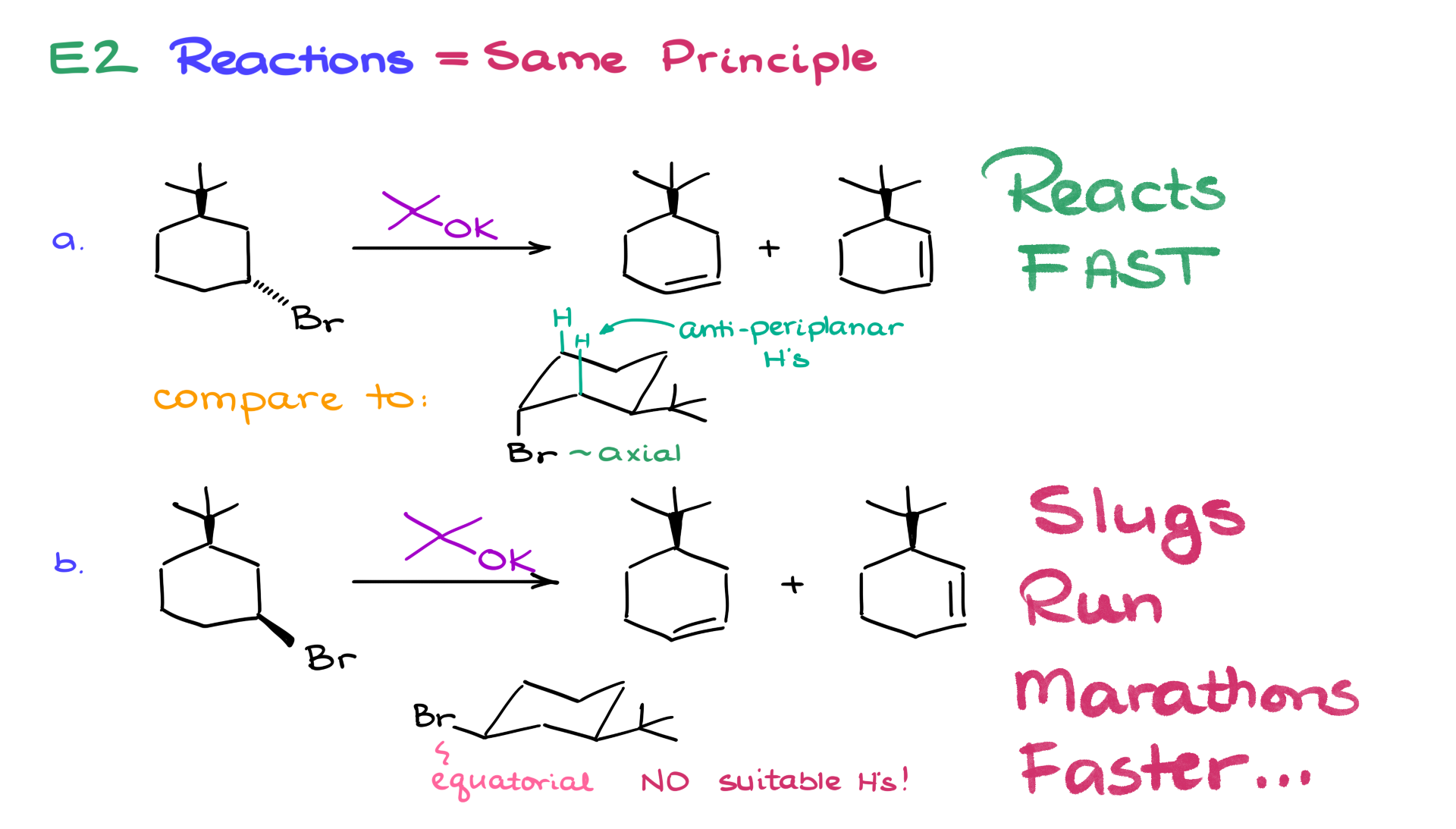
1. Reaction A: The most stable chair conformation places the leaving group in the axial position. For E2 reactions, both the leaving group and the beta hydrogen must be anti-periplanar, allowing perfect orbital alignment for elimination.
2. Reaction B: The leaving group is equatorial, and there are no suitable beta hydrogens in an anti-periplanar position. Thus, elimination is virtually impossible.
Reaction A proceeds rapidly, while Reaction B is extremely slow.
Key Takeaways

Here’s what to remember about chair conformations:
1. For SN2 reactions, the leaving group must be in the axial position to enable backside attack.
2. For E2 reactions, both the leaving group and the beta hydrogen must be axial and anti-periplanar.
3. Stability doesn’t always equate to reactivity. Factors like sterics and orbital alignment are critical.
In chair conformations, axial positioning is the king of reactivity, even though equatorial positions are more thermodynamically stable.
