Synthesis of Alcohols Using the Grignard Reaction
In this tutorial, I’ll show you how to tackle alcohol synthesis questions efficiently using the Grignard reaction.
Before we get started, note that I already have a dedicated tutorial on the Grignard reaction. If you need a refresher, check out that first. This tutorial focuses on using the Grignard reaction specifically for synthesis purposes.
Without further ado, let’s dive into our first example, where we’ll synthesize a target molecule using the Grignard reaction.
Understanding the Grignard Reaction
When a carbonyl compound reacts with a Grignard reagent, which is a strong nucleophile, the Grignard reagent attacks the carbonyl carbon. This attack pushes electrons onto the oxygen atom, eventually yielding an alcohol after protonation.

Types of Alcohols
- Primary Alcohols: Formed from formaldehyde.
- Secondary Alcohols: Formed from aldehydes (where one substituent is a hydrogen atom).
- Tertiary Alcohols: Formed from ketones.
In our initial example, we are targeting the synthesis of a secondary alcohol. But just knowing the reaction mechanism isn’t always helpful for exams. We need a practical method to identify the exact starting materials.
Algorithm for Identifying Starting Materials
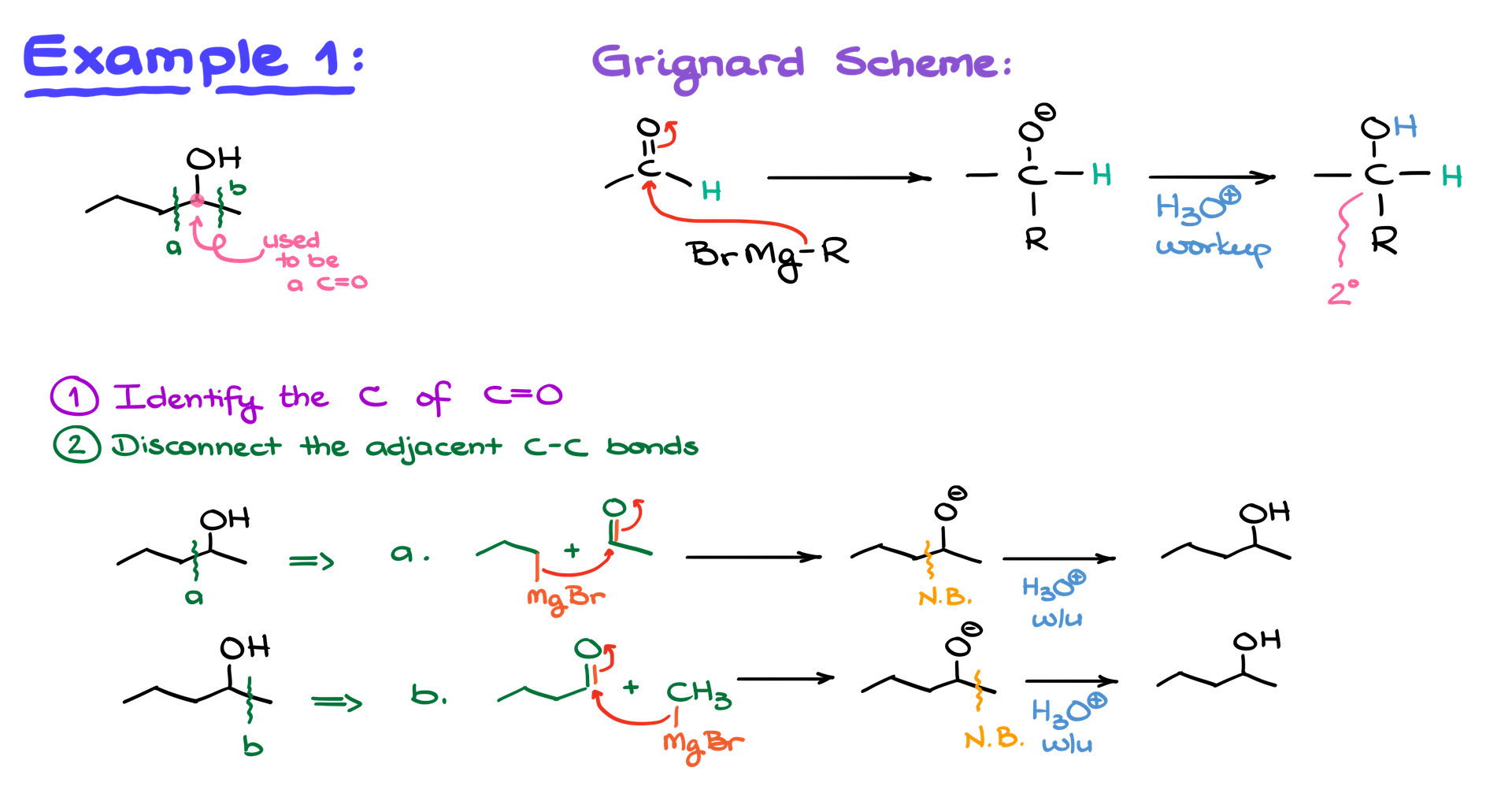
Here is a streamlined algorithm to help you identify the carbonyl compound and the Grignard reagent.
Step 1: Identify the Former Carbonyl Carbon
- Locate the carbon in your alcohol that used to be part of the carbonyl group. This will always be the carbon attached to the OH group.
- Next, disconnect the adjacent carbon-carbon bonds. We’ll label these as “Disconnect A” and “Disconnect B.”
Step 2: Redraw the Molecule
- For each disconnect, remove the selected bond and keep the oxygen attached to the former carbonyl carbon. The fragment with the oxygen becomes your carbonyl compound, while the other fragment becomes your Grignard reagent.
- Attach magnesium bromide to the carbon fragment to form your Grignard reagent.
Verification
React the carbonyl and Grignard reagent together. Ensure your carbon counts are accurate, as incorrect counting is a common mistake.
Tackling Complex Cases
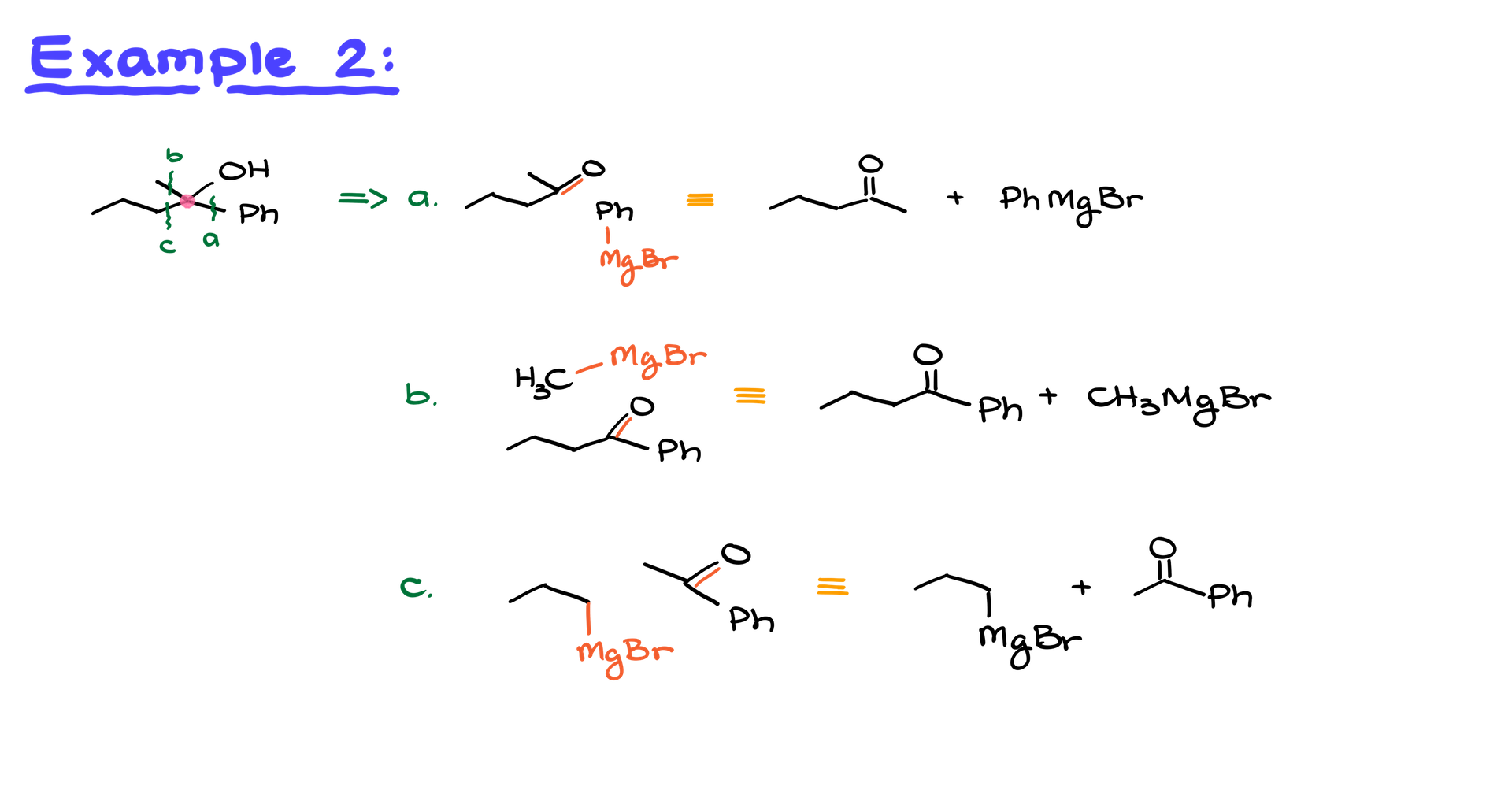
Now, let’s consider a more complicated example where a secondary alcohol is connected to three different groups: phenyl, propyl, and methyl. In this situation, we have three possible bonds to break, leading to “Disconnect A,” “Disconnect B,” and “Disconnect C.”
- Draw the original molecule.
- Erase each bond one at a time, convert the oxygen fragment into a carbonyl, and the other fragment into a Grignard reagent.
- Double-check carbon counts to ensure accuracy.
Choosing the Best Option
All possible combinations are correct from a theoretical perspective. However, practical constraints (like carbon limits) might favor one option over another. For instance:
- Tertiary Alcohols: Start with a ketone.
- Secondary Alcohols: Start with an aldehyde.
- Primary Alcohols: Use formaldehyde.
Example: If you react butyl magnesium bromide (a Grignard reagent with four carbons) with formaldehyde, you’ll form 1-pentanol after protonation.
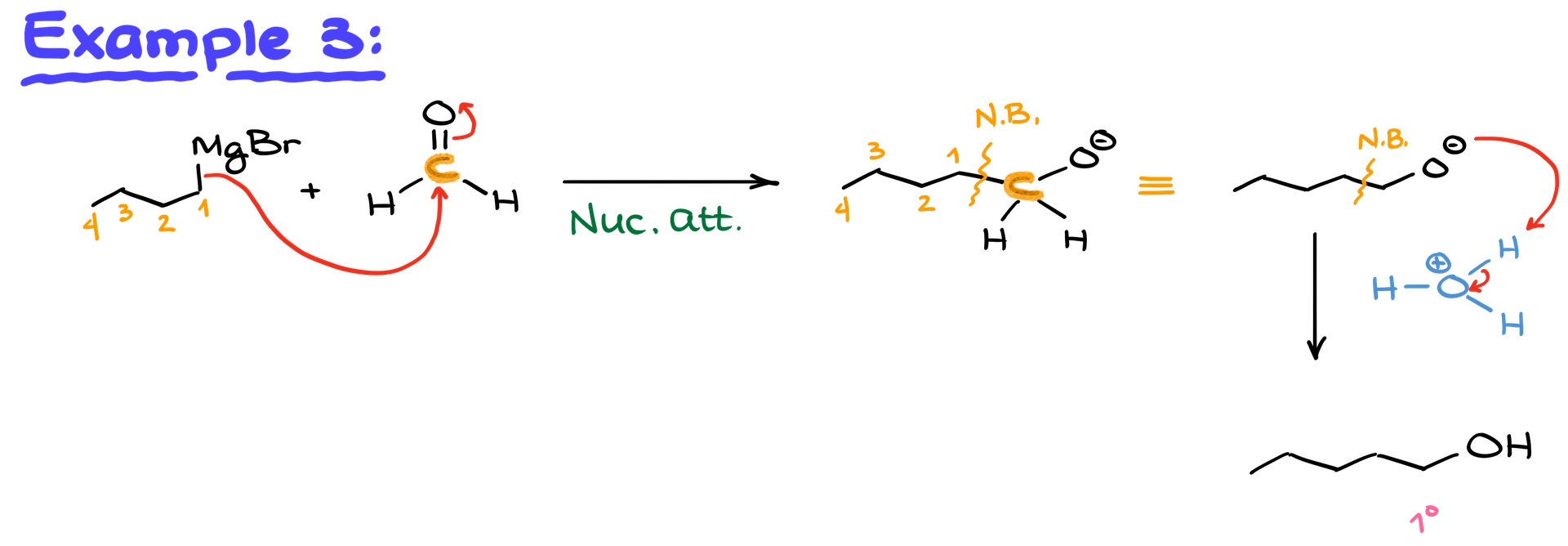
Using Epoxides for Alcohol Synthesis
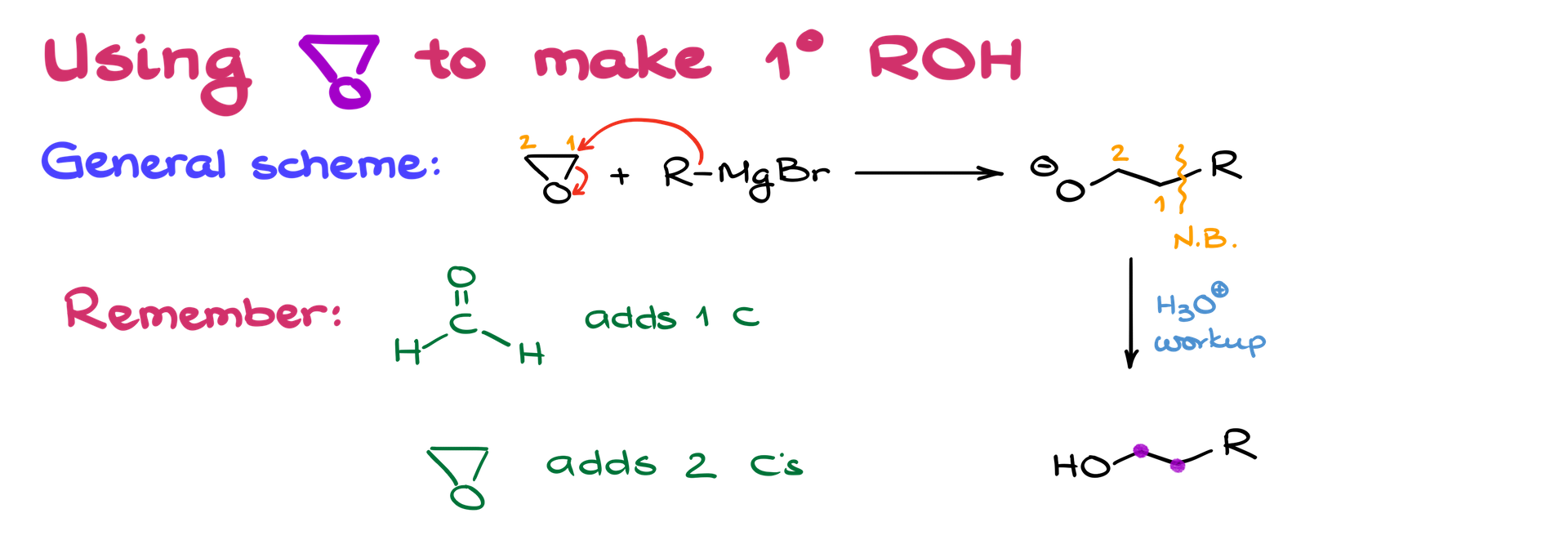
Besides carbonyl compounds, Grignard reagents can also open epoxides to form alcohols. This process adds two carbons to the chain, making it especially useful for primary alcohol synthesis. The Grignard reagent will attack the less substituted carbon in the epoxide.
Epoxide Selectivity
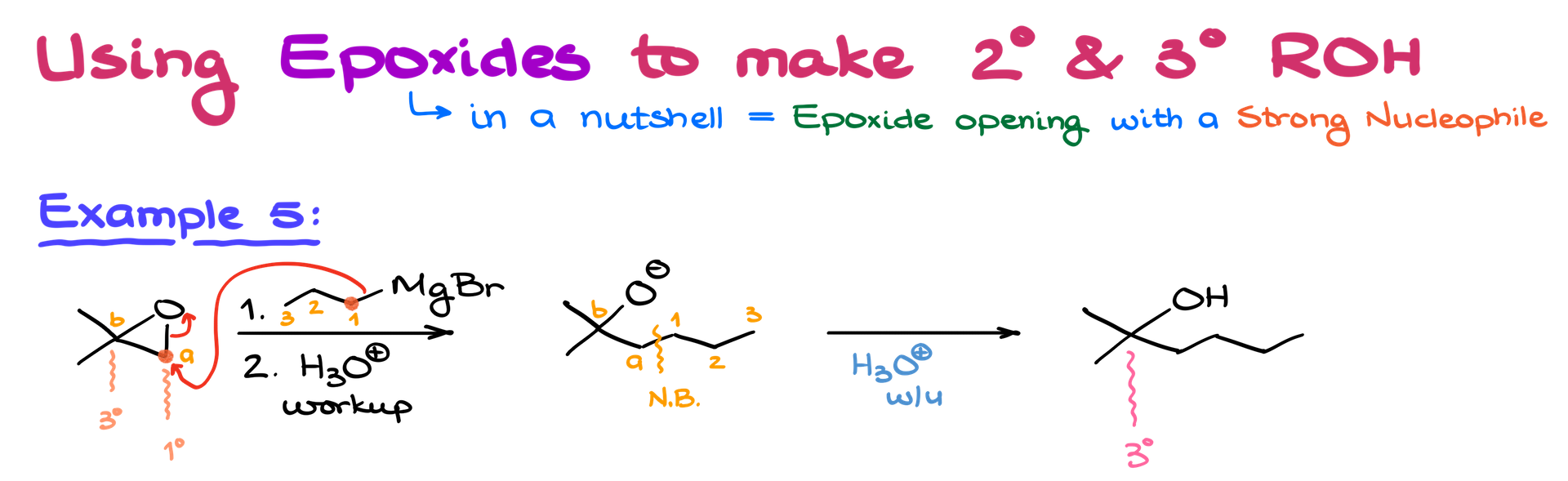
Always aim for regioselectivity: attack the less substituted carbon. For instance, if both carbons in the epoxide are secondary, the yield will be lower. Ideally, you want a primary or tertiary carbon.
Identifying Epoxide Precursors
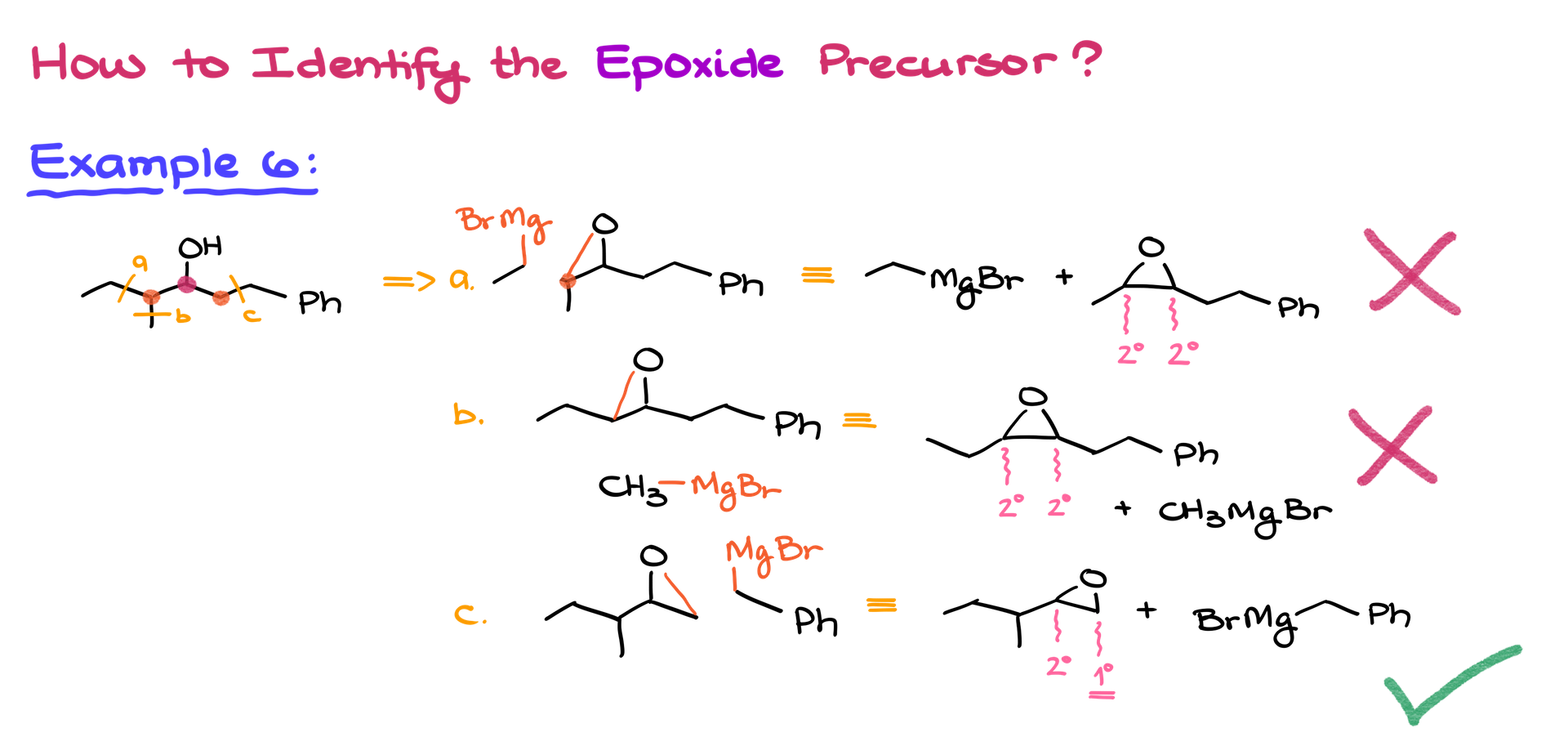
Identifying the correct epoxide precursor is critical, often trickier than finding carbonyl precursors. Use this approach:
- Identify the OH-bearing carbon.
- Consider adjacent carbons and test different disconnections.
- Ensure regioselectivity by selecting the right carbon to break.
Some combinations will yield poor selectivity, so be cautious. You need a clear distinction between more and less substituted carbons.
Cyclic Alcohol Synthesis
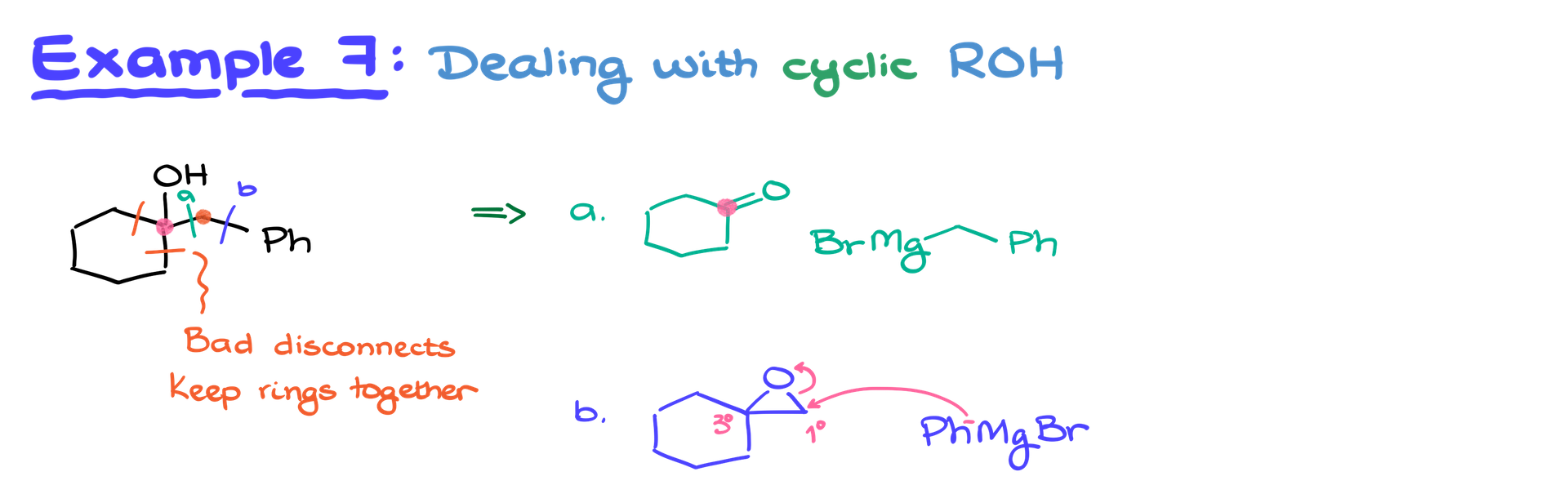
When synthesizing cyclic alcohols, keep the ring intact. Intramolecular Grignard reactions are uncommon due to complexity. Use the disconnect method thoughtfully:
- Identify bonds you can break without destabilizing the ring structure.
- Ensure accuracy in your carbon framework.
Trick for Acid Chlorides and Esters
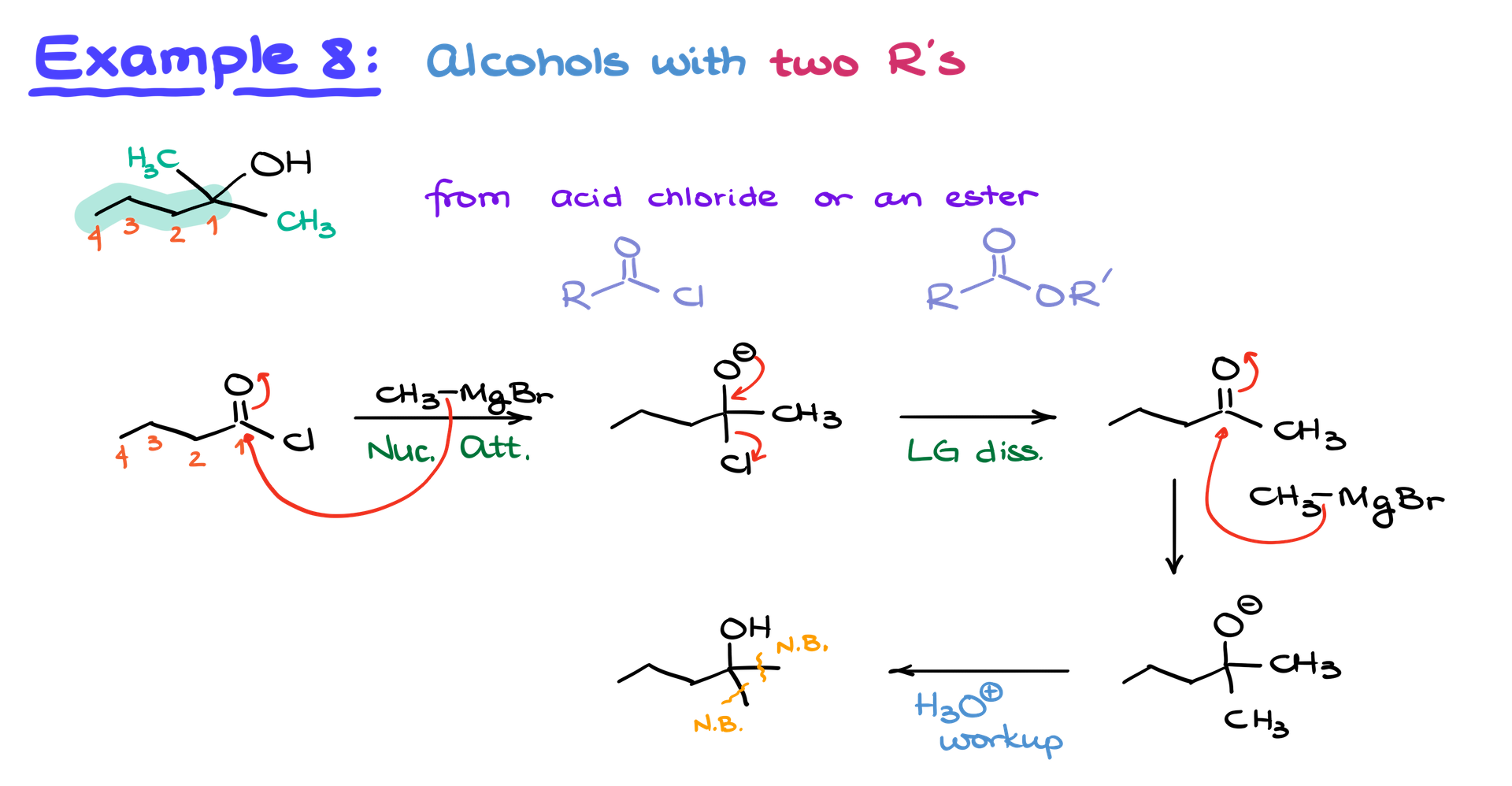
To make tertiary alcohols with identical substituents (like two methyl groups), use acid chlorides or esters. Highlight the main carbon chain, convert the alcohol to an acid chloride or ester, and then react with excess Grignard reagent. This method allows for efficient bond formation.
Example: If you have an alcohol with two methyl groups attached, convert the alcohol to an acid chloride and react with methyl magnesium bromide. The first equivalent forms a ketone, and the second completes the synthesis, yielding the tertiary alcohol.
Conclusion
The Grignard reaction is a powerful tool for synthesizing alcohols. Remember:
- Primary Alcohols: Start with formaldehyde.
- Secondary Alcohols: Start with aldehydes.
- Tertiary Alcohols: Start with ketones or use acid chlorides/esters.
Epoxides are also crucial but require careful handling and attention to regioselectivity. Be meticulous with your carbon counts and selective when breaking bonds. Use these strategies regularly, and you’ll be well-prepared for exams and practical applications.
