How to Find Stereoisomers
In this tutorial, we will go over how to easily determine if a molecule has stereoisomers by focusing on the key molecular features that give rise to stereochemistry. Instead of relying solely on definitions of enantiomers and diastereomers, which are helpful when comparing two molecules, we will learn to identify stereoisomers from the structure of a single molecule.
Key Sources of Stereochemistry
To determine if your molecule has stereoisomers, focus on these three primary sources of stereochemistry:
1. Chiral Atoms
2. Double Bonds
3. Cyclic Structures
Chiral Atoms
A chiral atom is an atom attached to four different groups. These atoms are a common source of stereoisomers in organic chemistry, typically found in carbon atoms, but potentially in other atoms like nitrogen, phosphorus, or sulfur.

For example:
• If a molecule contains a carbon atom bonded to four different groups, this carbon is chiral. As a result, you can draw two stereoisomers of the molecule. For instance, one stereoisomer may have chlorine on a wedge and hydrogen on a dash, while the other switches their positions. These are non-superimposable mirror images of each other, known as enantiomers.
Whenever you identify a chiral atom, there will always be a pair of enantiomers. If there are multiple chiral atoms in the molecule, the number of possible stereoisomers increases, up to a maximum of (where is the number of chiral atoms). However, this number may be reduced if some of the stereoisomers are meso compounds (achiral molecules with chiral centers).
Double Bonds
Double bonds, particularly those between carbon atoms, can also be a source of stereochemistry. For stereoisomers to exist, both carbons of the double bond must have two different groups attached.
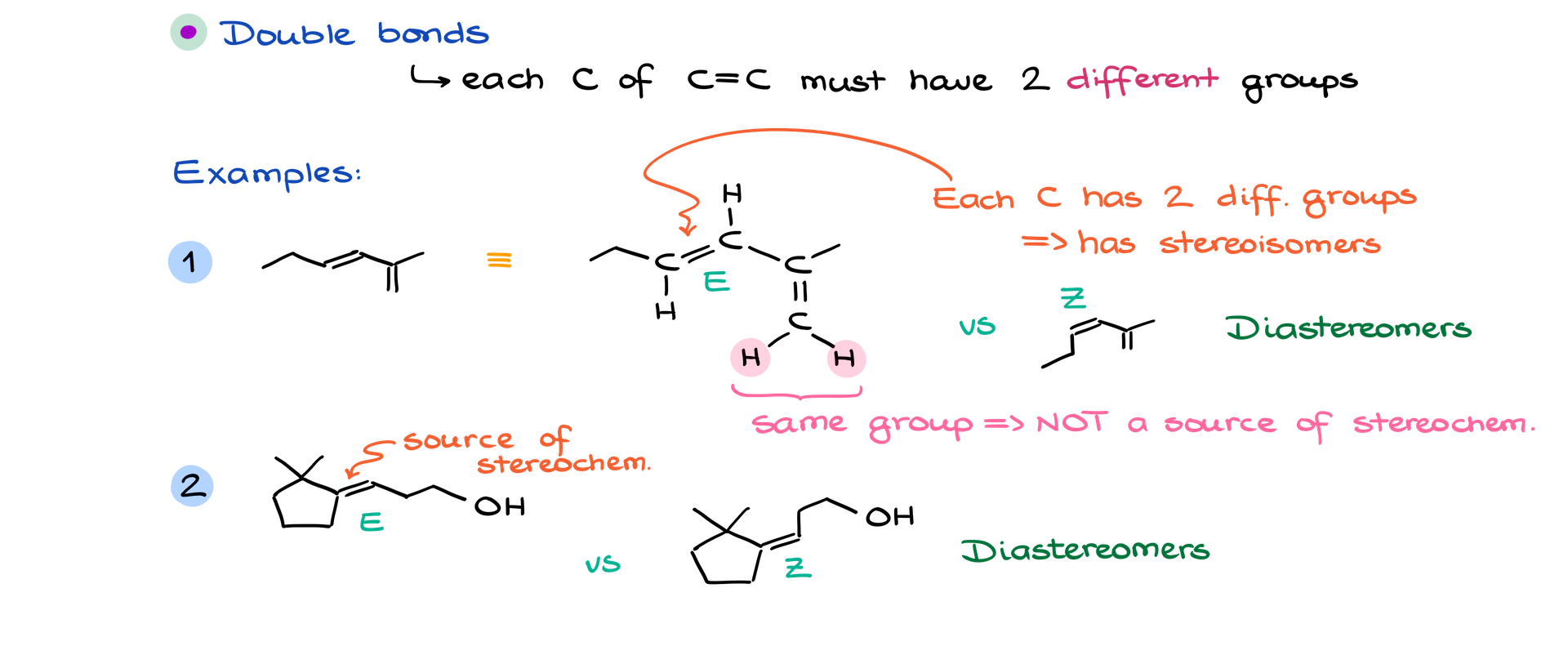
For example:
• In a molecule where one carbon of the double bond is attached to a hydrogen and an ethyl group, and the other carbon is attached to a hydrogen and the rest of the molecule, this double bond is stereogenic. You can have two configurations: E (opposite side) or Z (same side), depending on the relative positions of the substituents. These two forms are diastereomers because they are not mirror images.
Not all double bonds will create stereochemistry—only those where each carbon has two different groups.
Cyclic Structures
Cyclic structures, particularly disubstituted rings, are another source of stereochemistry. Even if the individual atoms in the ring are not chiral, the relative positions of substituents on the ring can create stereoisomers.
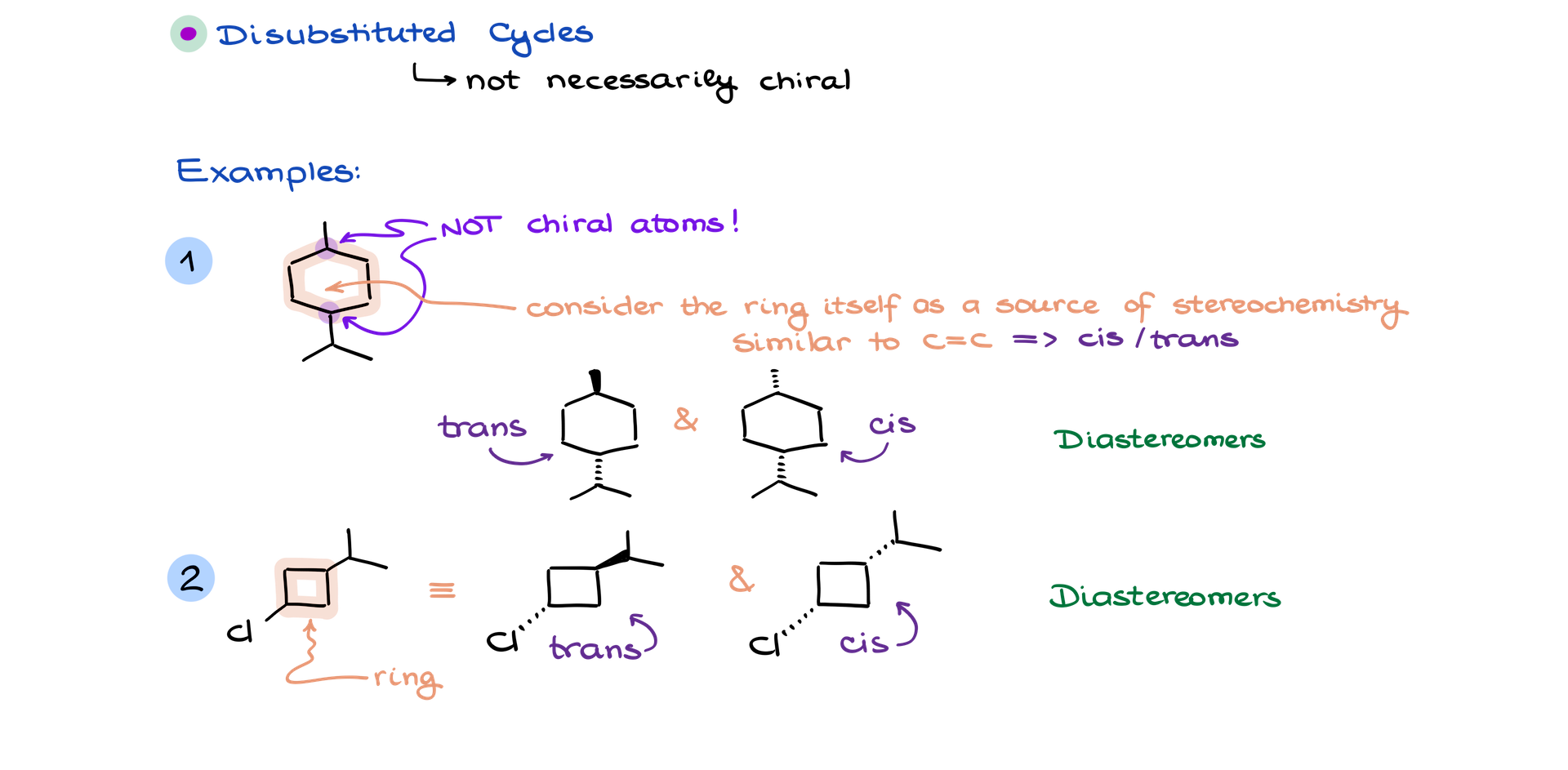
For example:
• In a disubstituted cyclic molecule, if the two substituents (like a methyl group and an isopropyl group) are on the same side of the ring, the molecule is a cis isomer. If they are on opposite sides, it is a trans isomer. These are also diastereomers because they are non-superimposable and not mirror images.
Importantly, even if a cyclic molecule has a plane of symmetry and no chiral atoms, it can still produce stereoisomers based on the arrangement of substituents.
Step-by-Step Guide to Identifying Stereoisomers

1. Look for Chiral Atoms: Identify any atoms with four different groups attached. If present, the molecule will have at least one pair of enantiomers. If there are multiple chiral centers, calculate the potential number of stereoisomers using .
2. Check Double Bonds: For each double bond, ensure that both carbons have two different groups. If so, the bond may create E/Z isomers, which are diastereomers.
3. Examine Cyclic Structures: For cyclic molecules, determine whether the substituents on the ring are on the same or opposite sides, leading to cis/trans isomers, another form of diastereomers.
Concluding Thoughts
Identifying whether your molecule has stereoisomers is straightforward if you know what to look for. By focusing on chiral atoms, double bonds, and cyclic structures, you can easily determine whether stereoisomers are present. Keep these features in mind, and you’ll be well-prepared to analyze stereochemistry in your molecules.
