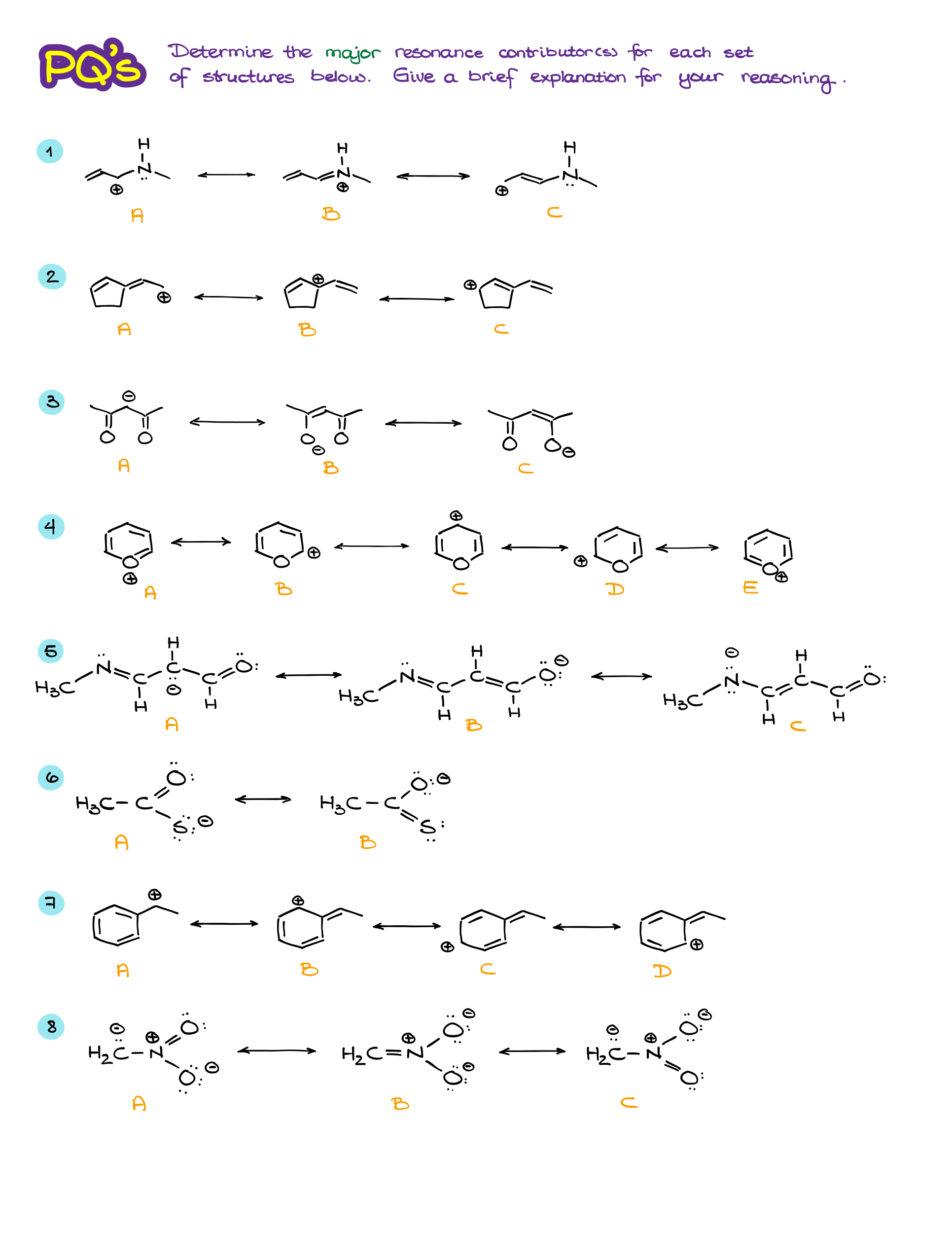
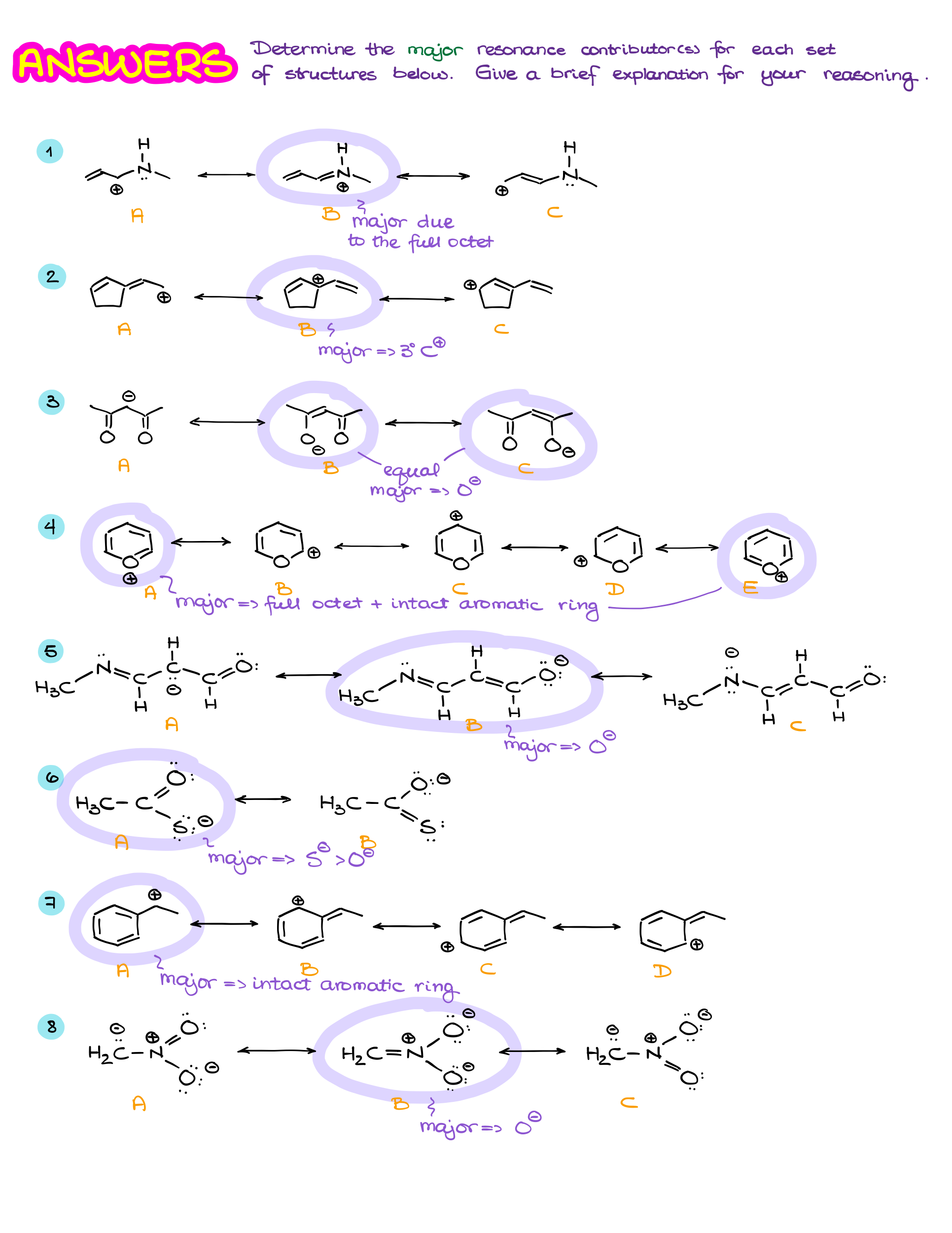
Major vs Minor Resonance Contributors
Resonance is one of those topics in organic chemistry that can trip up students, but it’s absolutely crucial for understanding how molecules behave. I have already talked about the resonance structures in another tutorial. If you haven’t worked through that one yet, di it first before looking at the material here. In this tutorial, I’ll go over the most common questions students have about resonance and pay close attention to the major and minor resonance contributors. So, let’s break it down step by step and answer some common questions that often come up.
Why Do We Care About Resonance?
Resonance helps us understand how electrons are distributed in a molecule. In many cases, a single Lewis structure can’t fully capture what’s happening with the electrons. So, resonance gives us a way to represent the true electron distribution as a hybrid of multiple possible structures. This is key because it affects a molecule’s stability, reactivity, and bond lengths. Essentially, resonance shows how delocalized electrons can make a molecule more stable and influence how it reacts with other molecules.
What’s the Difference Between Resonance Structures and the Resonance Hybrid?
This is where things can get confusing, but it’s actually pretty simple once you get the hang of it.
- Resonance structures are different ways of drawing the arrangement of electrons (like lone pairs or pi bonds) in a molecule, while the atoms stay in the same positions. These structures are hypothetical—they don’t exist on their own but help illustrate possible electron placements.
- The resonance hybrid, on the other hand, is the real deal. It’s the true form of the molecule, which is an average or “blend” of all the resonance structures. The hybrid reflects the actual distribution of electrons, where they’re spread out (delocalized) over several atoms, rather than being confined to one bond or location.
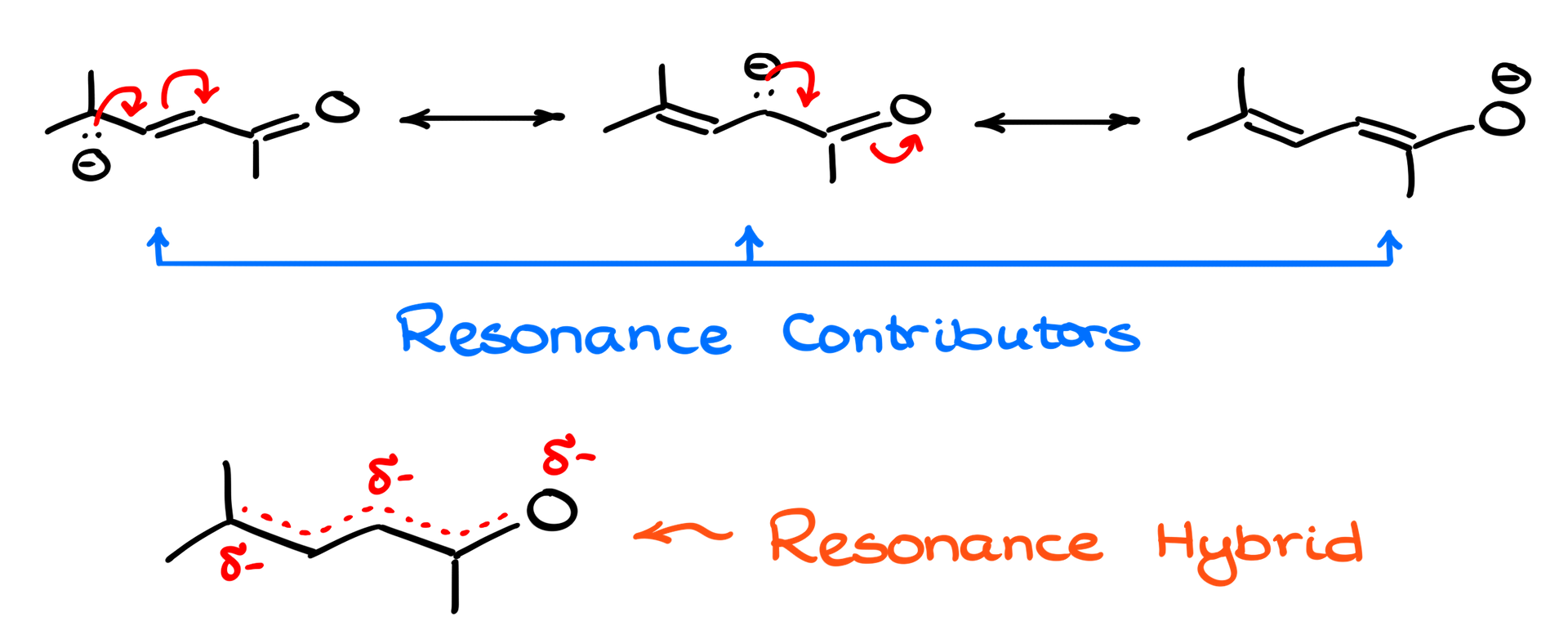
Think of the hybrid as a “best fit” representation of the molecule, where the electrons are more spread out and the molecule is more stable as a result.
Why Not Just Draw the Resonance Hybrid Instead of the Resonance Structures?
You might wonder, “If the hybrid is closer to reality, why don’t we just draw that?” Well, it’s because the hybrid doesn’t clearly show how electrons are shifting or delocalized. Resonance contributors, on the other hand, help us visualize specific electron movements, like lone pairs shifting or pi bonds relocating. This is useful for predicting how the molecule will react with others.
By showing each resonance contributor, we can better understand the nature of electron delocalization, making it easier to predict a molecule’s stability and reactivity. So, even though the hybrid is the real electron distribution, resonance contributors are the practical tool that gives us insight into how electrons are behaving.
Are All Resonance Contributors Equal?
Nope! Not all resonance contributors are equally important. Some are more significant because they more closely resemble the true structure of the molecule. Here are a few rules to determine which contributors matter the most:
- Complete octets: The more atoms that have a full octet of electrons, the better. We especially pay attention to the atoms bearing charges.
- Carbocations: If we only have structures with carbocations, we’ll typically prioritize the 3° position over the 2° and 1°
- Charge location: If a contributor has charges, negative charges are best on electronegative atoms like oxygen or nitrogen.
- Minimized formal charges: Contributors with fewer formal charges are more stable. Neutral molecules tend to be more significant.
- Aromatic Rings: Intact aromatic rings tend to make larger contribution to the overall hybrids than the “broken” aromatic rings that donated some of the electron density to make a resonance contributor.
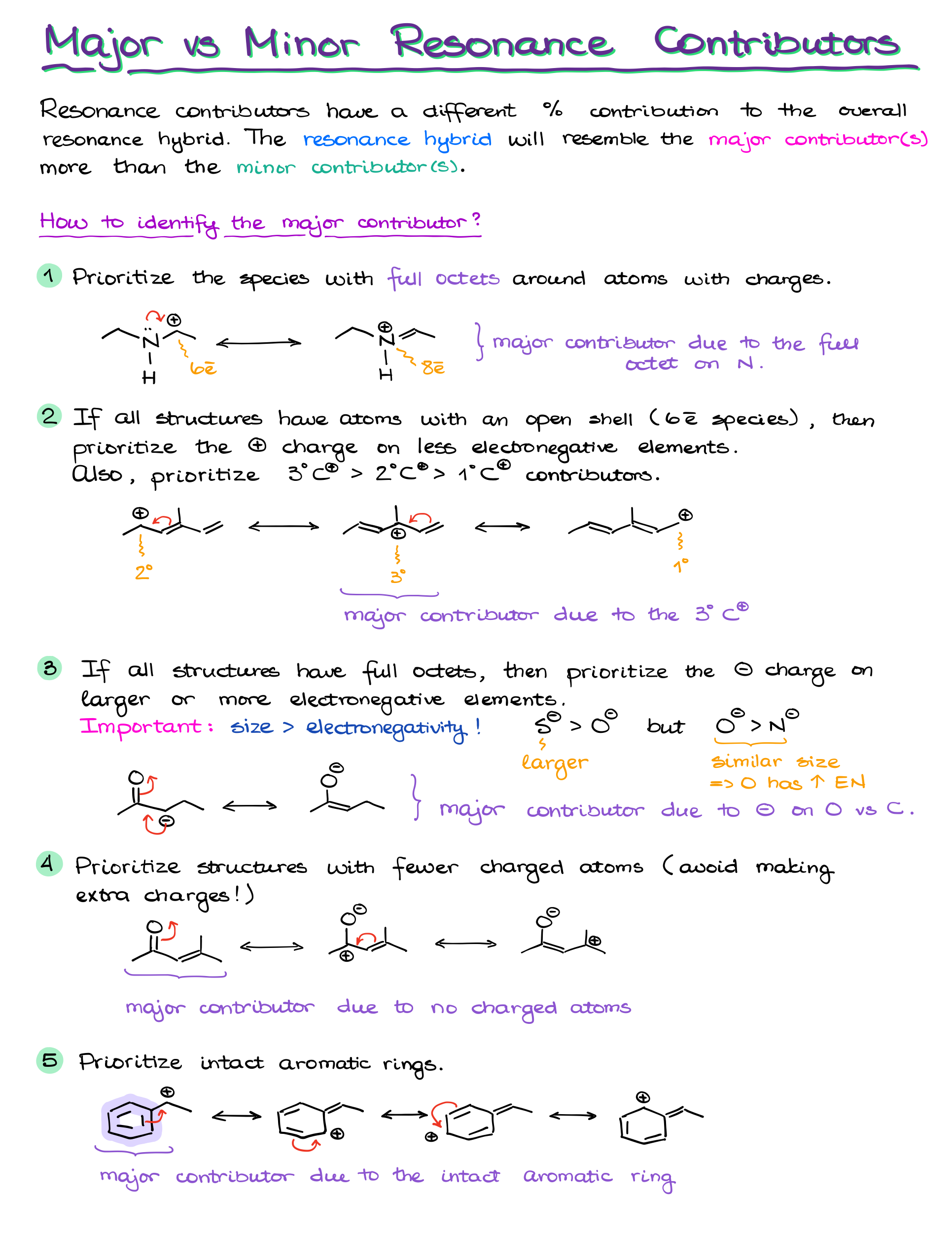
So, while having multiple resonance structures is good, the most important ones will follow these rules. These contributors make a bigger impact on the overall stability and properties of the molecule.
Wrapping It Up
Resonance isn’t just about counting how many contributors a molecule has—it’s about identifying the most stable and significant ones that help us understand how the electrons are really distributed. Once you get the hang of spotting the important resonance structures, the concept of resonance starts to make a lot more sense. It’s all about understanding how delocalized electrons stabilize molecules and affect their behavior.
Practice Questions