Synthesis and Reactions of Acid Chlorides
In this tutorial we’re going to go over the acid chlorides (acyl chlorides), their synthesis, and common reactions of acid chlorides you’re likely going to see in your homework or on the test.
First of all, what exactly are the acid chlorides?
Acid chlorides, or acyl chlorides, are derivatives of carboxylic acids that have a chlorine atom directly attached to the carbonyl group.

Due to the polarization of the carbonyl and inductive effect of the chlorine atom, acid chlorides are quite electrophilic and are extremely reactive towards nucleophiles. While there are a lot of possible reactions of acid chlorides, here I’m going to only focus on the most common ones that are typically covered in a sophomore organic chemistry course.
Synthesis of Acid Chlorides
Before we can look at the reactions of acid chlorides, it’s a good idea to first look at how exactly we make them. There are two typical reactions you’re going to see that yield acid chlorides:
- Reaction of carboxylic acids with thionyl chloride,
- Reaction of carboxylic acids with PCl3 or PCl5.
The reaction with thionyl chloride is more common, so let’s look at it first.
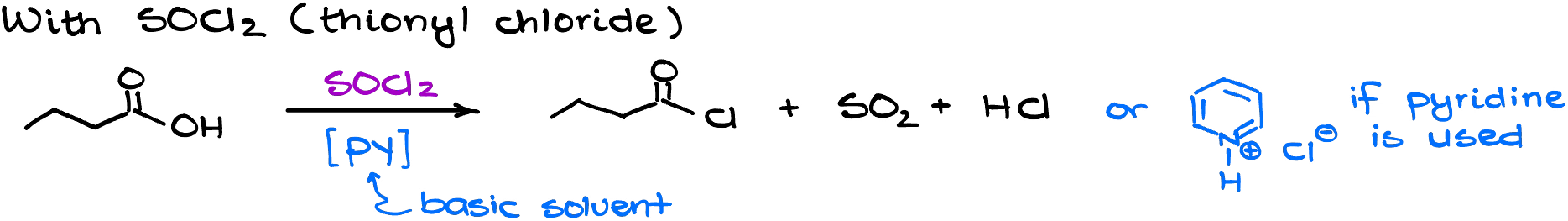
Mechanistically speaking, this reaction is a somewhat typical acyl substitution. The first part of the mechanism modifies the carboxylic acid so that we have a good leaving group. Once we have a good leaving group on the carbonyl, we can do the acyl substitution reaction making the acid chloride.
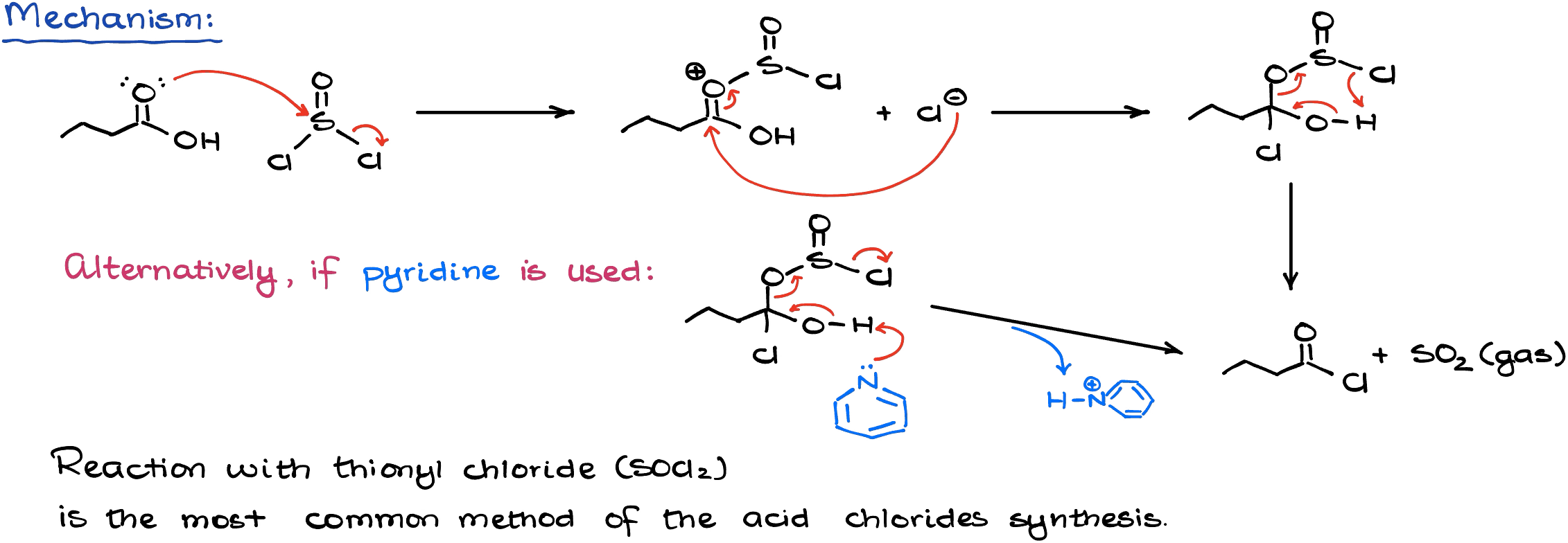
Importantly, the co-products in this reaction are either gaseous or are bound by pyridine if we choose to use that as a solvent. The use of pyridine here is completely unnecessary. However, we often see it in the textbooks and it’s likely your instructor is going to give you this reaction with pyridine as a solvent.
The second method uses phosphorous tri- or pentachloride. This reaction is based on the same principle as the former one — we make a good leaving group and kick it out.
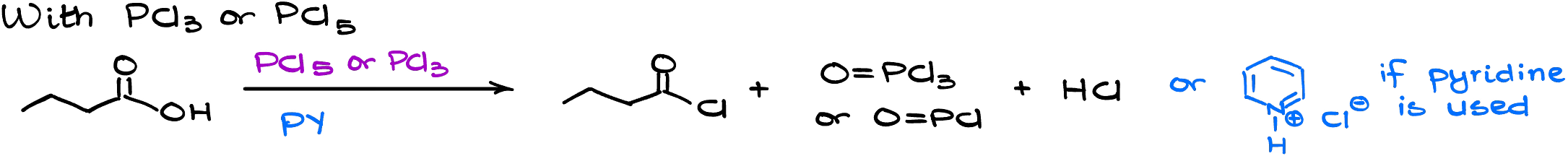
Mechanistically, this reaction starts in a similar way to the former reaction with thionyl chloride by using our reagent to modify the carboxylic acid. I’m only going to show the mechanism here for the phosphorous pentachloride, but the phosphorous trichlorde mechanism is functionally the same.
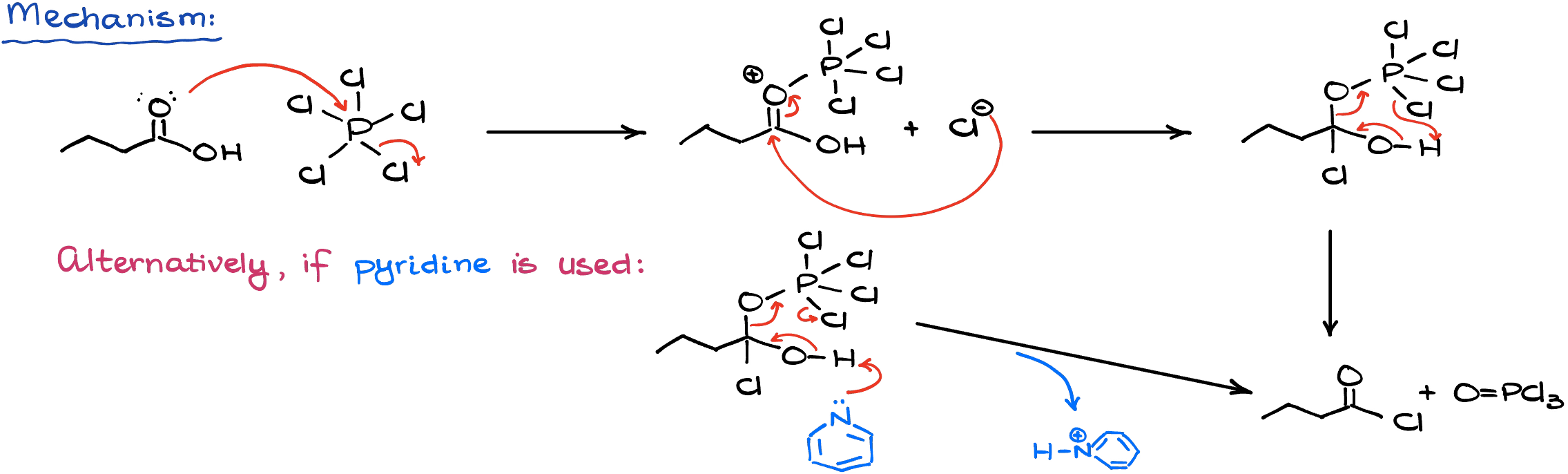
The co-product here is unfortunately not a gas, so it’s a little more difficult to isolate our product.
Which method should you choose to use on the test? It doesn’t matter. You may choose any. As I’ve mentioned a moment ago, the reaction with thionyl chloride is more common. So, chances are, your instructor didn’t even show you the ones with phosphorous chlorides at all.
Reactions of Acid Chlorides
Acid chlorides are among the most electrophilic derivatives of carboxylic acids.

So, it’s easy to convert acid chlorides into virtually any other carboxylic acid derivative by replacing the chlorine atom with the corresponding nucleophile. So, let’s look at these reactions one by one from more electrophilic derivatives, to the less electrophilic ones.
All these reactions will have the same general mechanism:
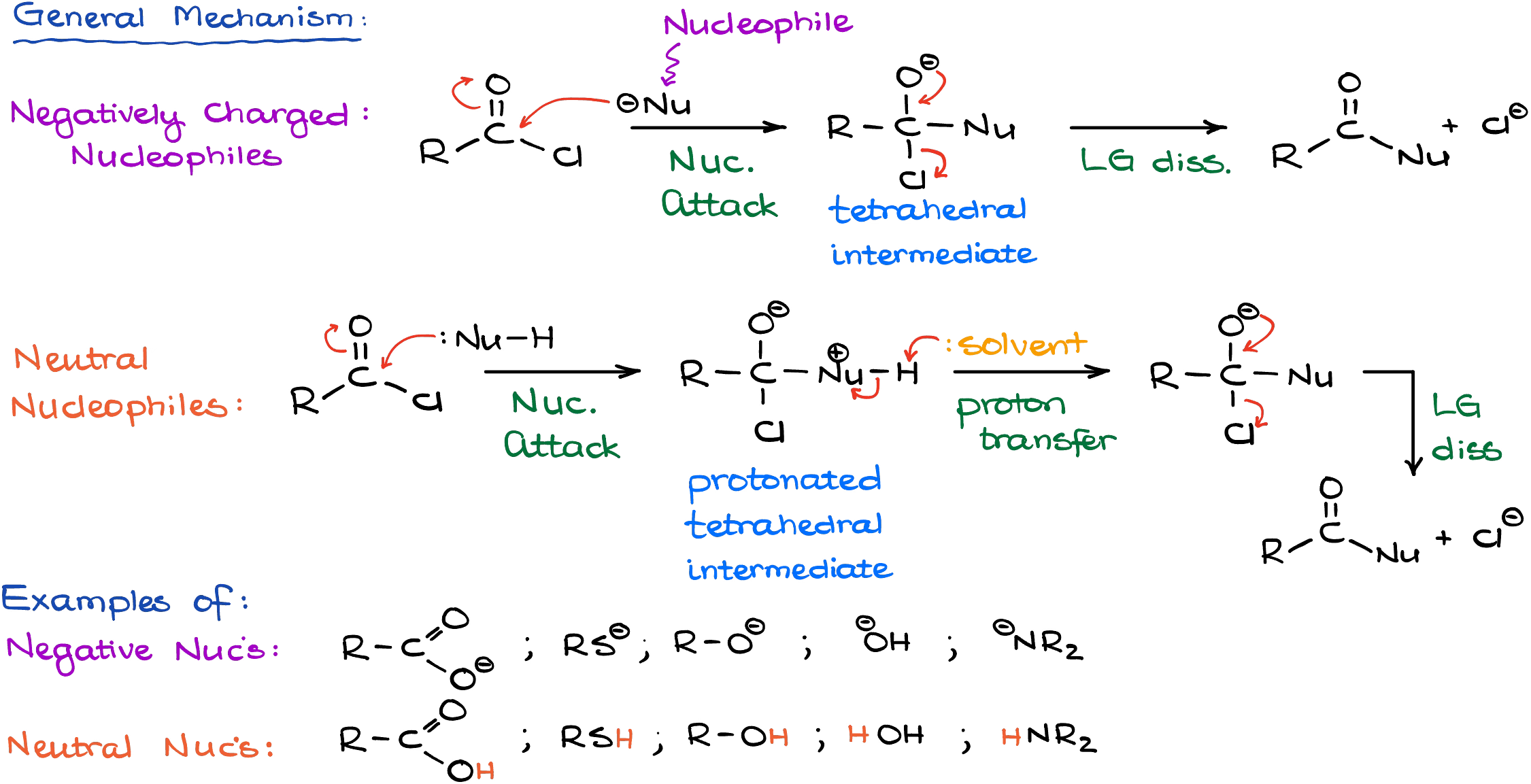
The first step is always going to be a nucleophilic attack on the carbonyl of the acid chloride making a tetrahedral intermediate. Depending on the nature of your nucleophile, you might need a proton transfer. Then, we’re going to have a leaving group dissociation giving us the final product.
Reaction with Carboxylic Acids
When acid chlorides react with carboxylic acids, they make the corresponding acid anhydrides.
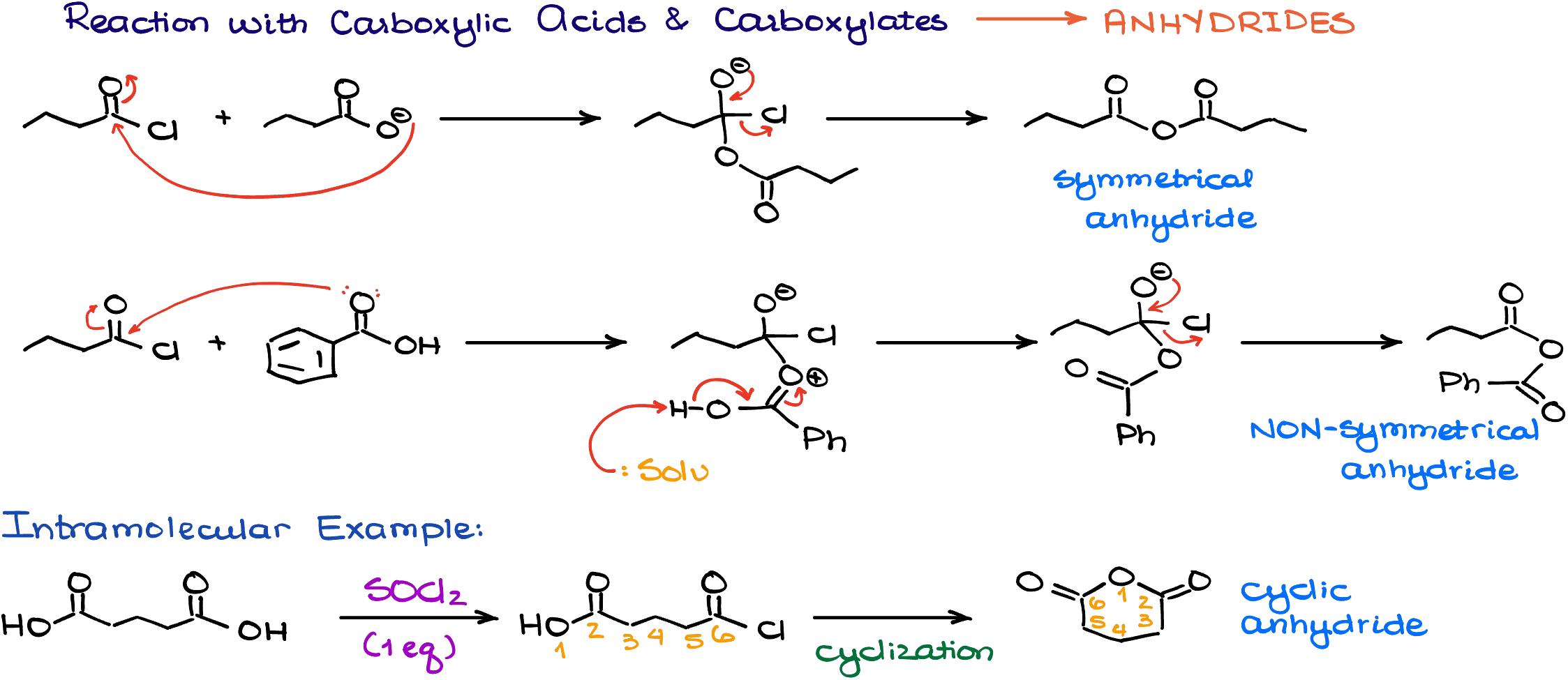
The reaction occurs very smoothly with either deprotonated version (carboxylate) or the neutral version of the carboxyl acid itself. As the result, you can make either symmetrical or non-symmetrical acid anhydrides. Some instructors like to make asymmetric anhydrides on the tests, even though they are not particularly useful for any purposes.
Reaction with Alcohols
When acid chlorides react with alcohols or alkoxides, they make corresponding esters.
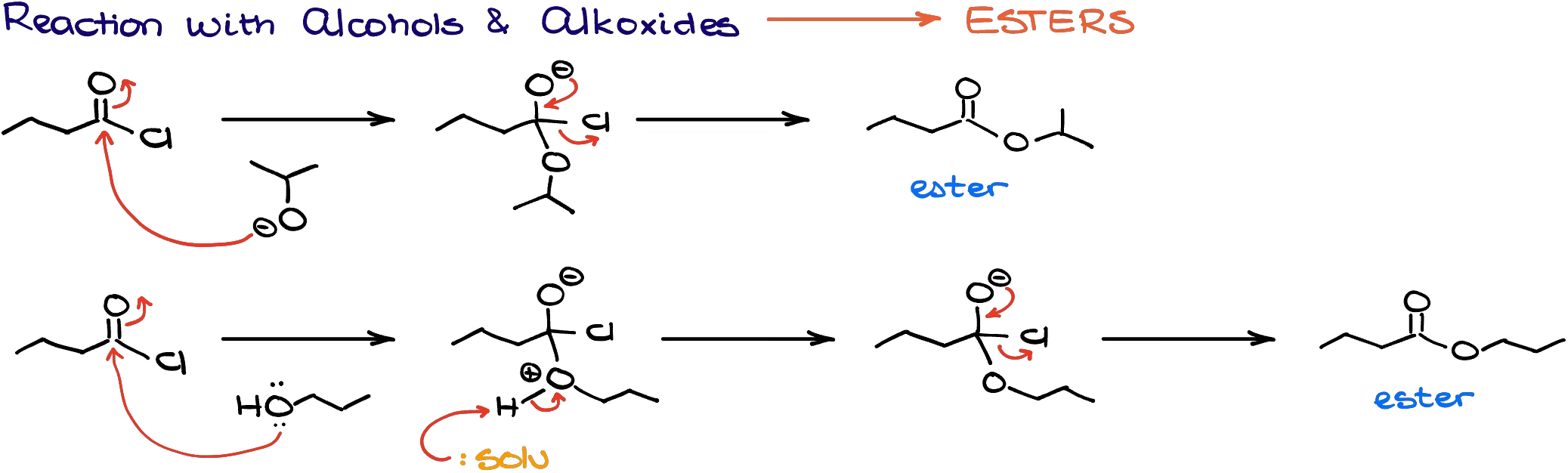
Typically, we perform this reaction with alcohols rather than alkoxides. Alkoxides are quite reactive and bringing them together with acid chlorides, which are also very reactive, make a very exothermic reaction which is hard to control. Plus, alkoxides are basic which may also result in unwanted side-reactions.
Hydrolysis of Acid Chlorides
When acid chlorides react with water or hydroxides, they make carboxylic acids.
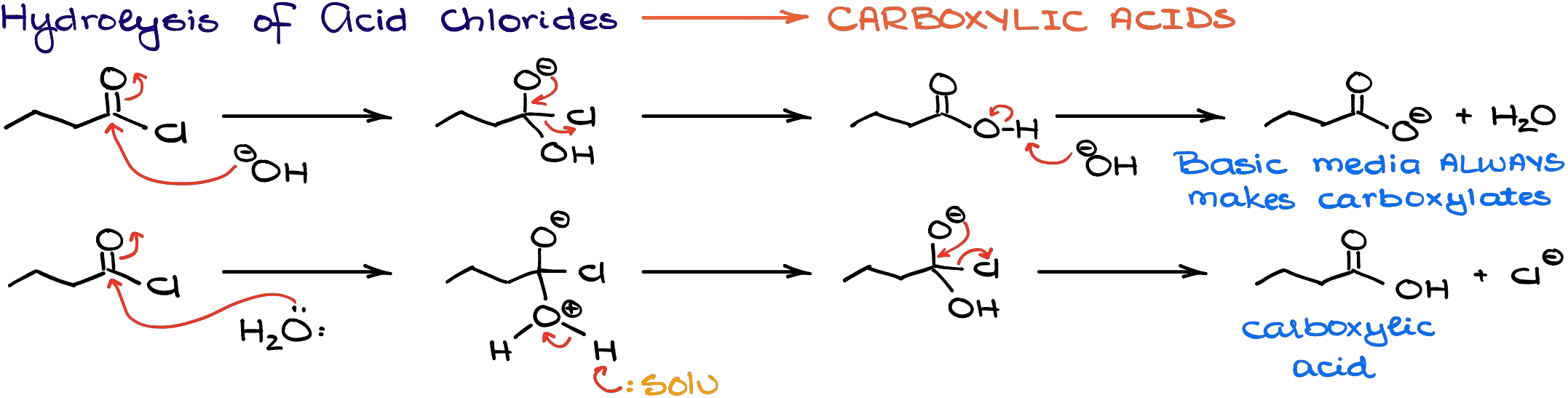
Like in the case of alkoxides, we typically don’t do this reaction with hydroxides. Additionally, reaction with hydroxides results in deprotonation of the carboxylic acid making a carboxylate (salt) with whatever base we’re using.
Reaction with Amines
Reaction of acid chlorides with amines results in the formation of the amides.
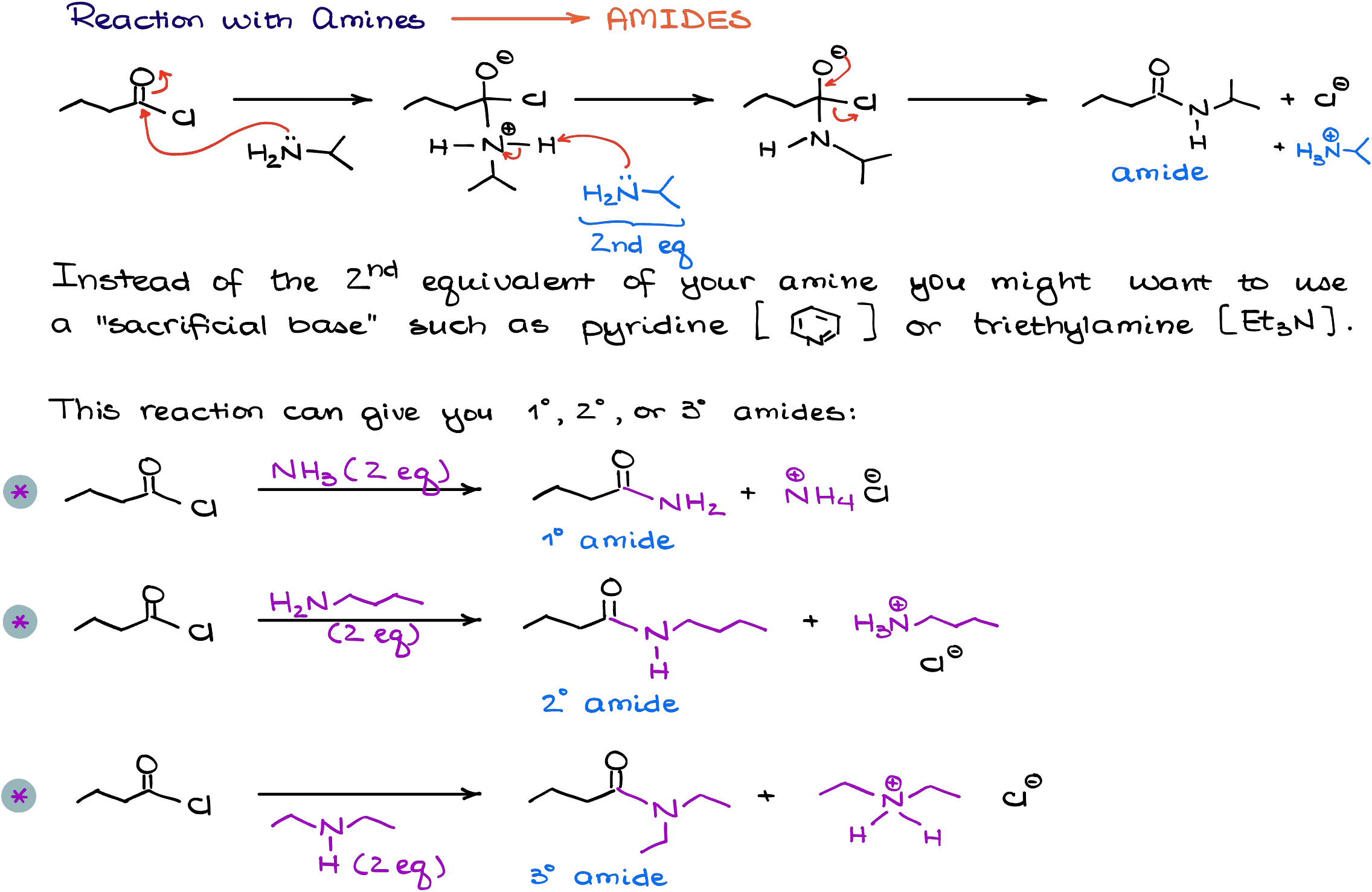
This reaction requires either two equivalents of the amine or a sacrificial base that is required to neutralize hydrochloric acid (co-product in this reaction). As a “sacrificial base” we typically use either triethylamine or pyridine. This becomes necessary if your amine is very expensive and you can’t afford to just waste one equivalent of the reagent.
Reactions with Organometallic Reagents
Acid chlorides react with organometallic compounds like organolithium compounds, Grignard reagents, and organocuprates (Gilman reagent) giving alcohols or carbonyls depending on the reaction conditions and the nature of the organometallic compound.
Organolithium compounds and the Grignard reagents react with acid chlorides in a similar fashion. The reaction typically goes through two rounds of addition.
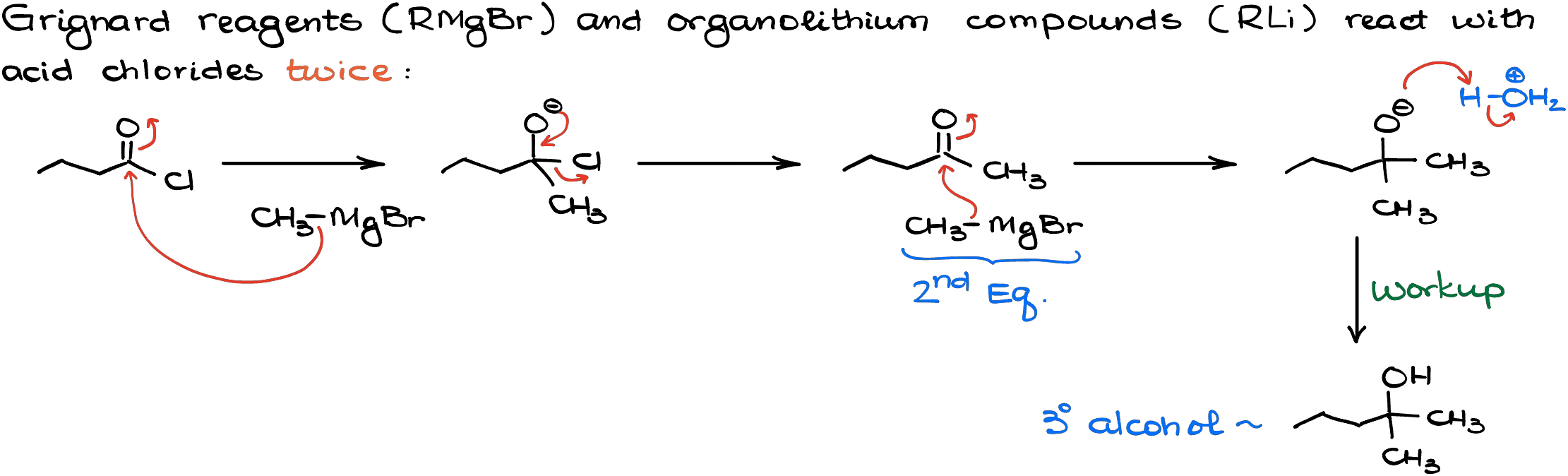
However, as the intermediate in this reaction — ketone — is less reactive than the acid chloride, it is possible to neatly control your reaction and only perform a single addition. This can only be done if we add the organometallic reagent to our acid chloride to make sure that the chloride is always in excess. But even then, the reaction tends to be less and less controllable as we consume the acid chloride and make more and more ketone.

So, unless your instructor specifically points out that this is ok for your class, always do the addition twice like in the first example I showed you here.
Is it possible to make sure we stop at the formation of the ketone though? Yes! Gilman reagents (organocuprates) do not react with ketones making it possible to stop the reaction at the formation of the ketone.
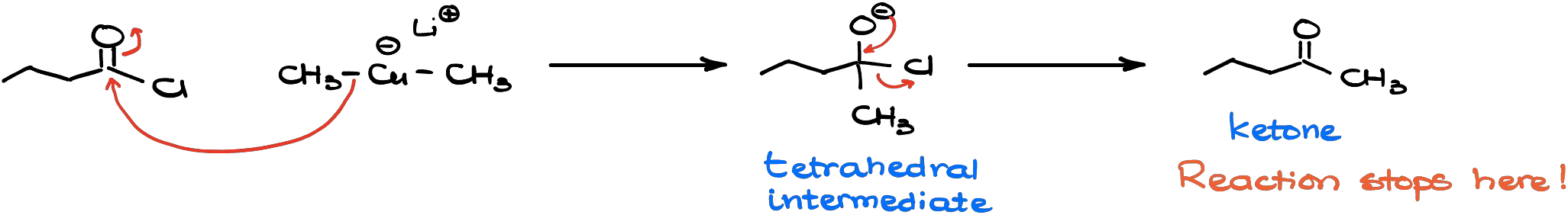
So, if you want to make sure that you only do a single addition of your organometallic reagent to the acid chloride, use the organocuprates.
Reduction of Acid Chlorides with Complex Hydrides
Complex hydrides are excellent sources of nucleophilic hydride ions. Since acid chlorides are very electrophilic, they easily react with all common complex hydrides like lithium aluminum hydride or sodium borohydride.
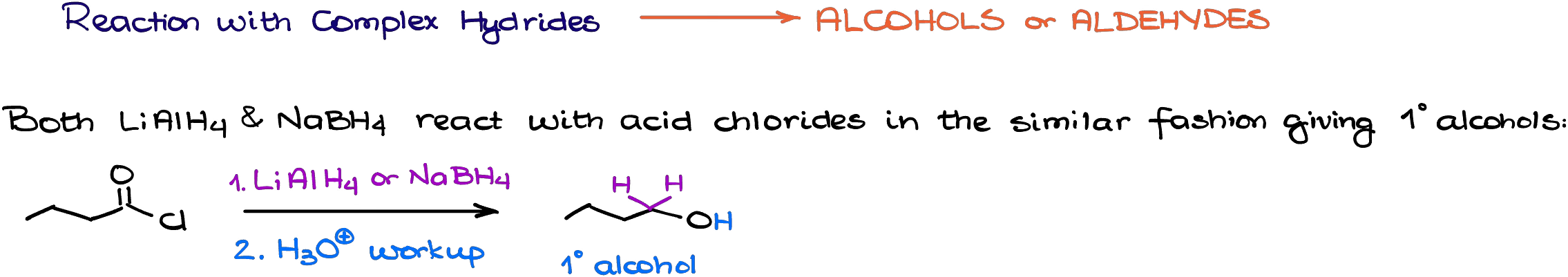
An important thing to remember here is that these reactions will always give you a complete reduction all the way to the corresponding primary alcohols. Mechanistically speaking, the reaction is just a series of the nucleophilic attacks on the carbonyl.
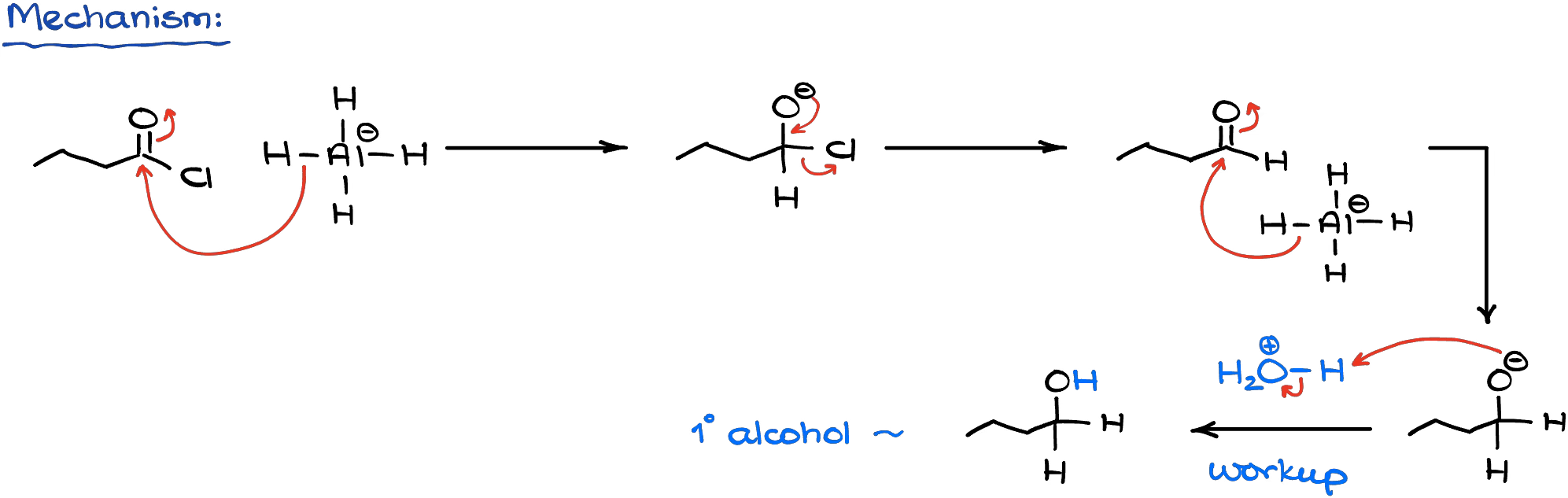
And as the hydrides are intrinsically basic, we are always going to end up with the alkoxide as the product. So, we need to make sure we do the acidic workup at the end to neutralize (protonate) the alkoxide.
Interestingly enough, but if you use a very bulky complex hydride at a very low temperature, you can reduce the acid chloride to an aldehyde and not an alcohol. The most common examples of such bulky complex hydrides are lithium tris-tert-butoxy aluminum hydride and diisobutylaluminum hydride (DIBAL).
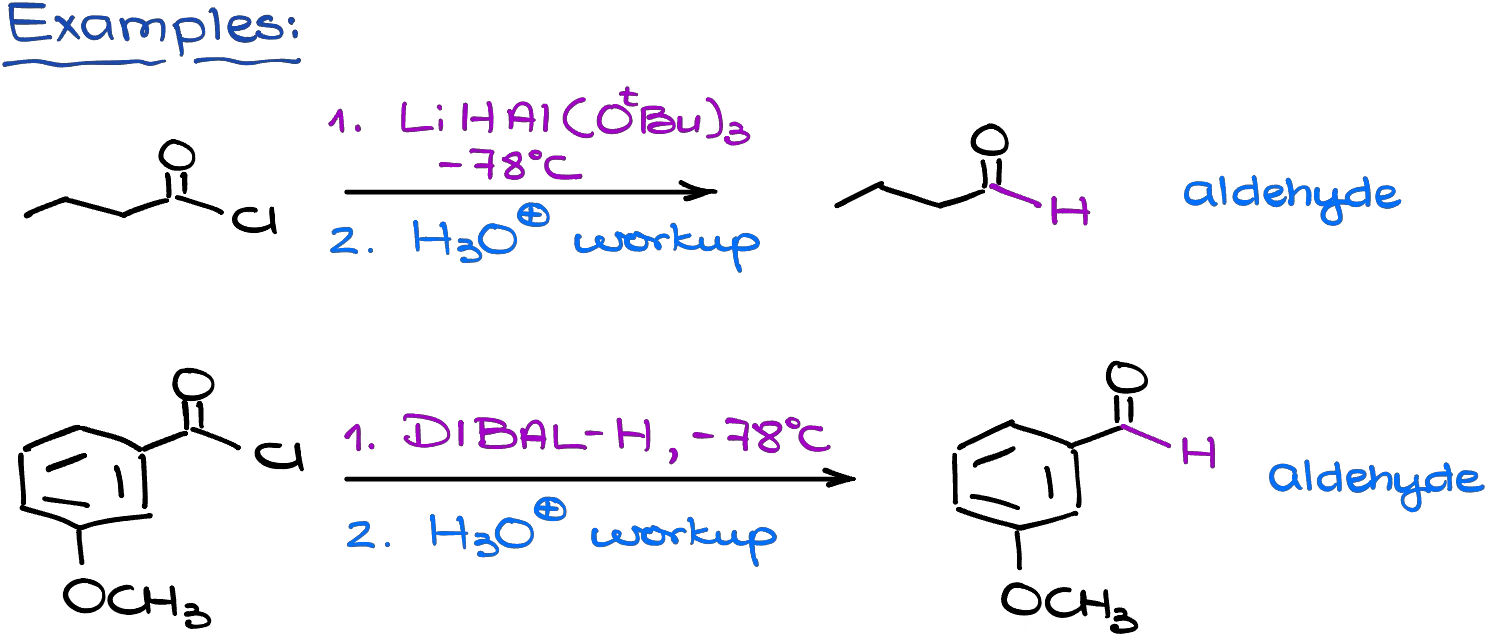
A low temperature here is essential. If the temperature is not low enough, the bulky hydrides are going to act just like the regular hydrides and reduce your acid chloride all the way to the primary alcohol.
