Synthesis of Carboxylic Acids
In this tutorial, I wanna talk about the various ways we make carboxylic acids in organic chemistry. And while there are a lot of different synthetic pathways that can result in the formation of a carboxylic acid, here I wanna focus on the most common reactions you’re going to see in your course. These are also going to be the most common reactions your instructor is going to expect you to use in your multistep syntheses.
Two Ways to Approach the Carboxylic Acid Synthesis
There are two ways how I’m going to be classifying the reactions that give us carboxylic acids:
- Reactions that preserve the number of carbons in our molecule, and
- Reactions that either add or remove carbons from our starting material.
Oxidation of Primary Alcohols
The first type is fairly straightforward and consists of one reaction — oxidation of primary alcohols.
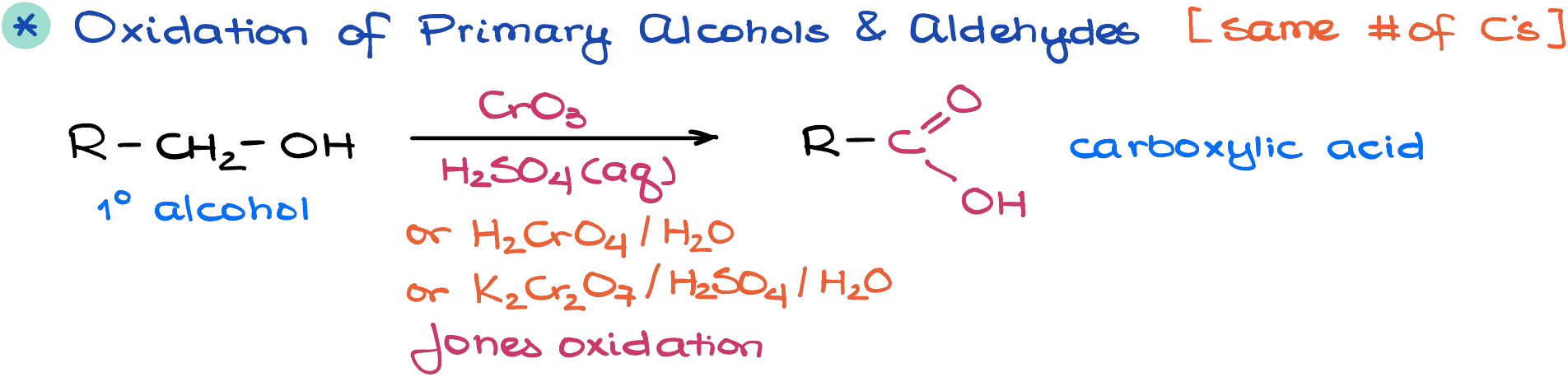
The typical oxidizing reagent that we’re going to use in this reaction is the Jones reagent (CrO3 + aqueous H2SO4) or any variation of it. The important thing to keep in mind here though, is that the reaction is not selective and will oxidize all eligible alcohols in this reaction.
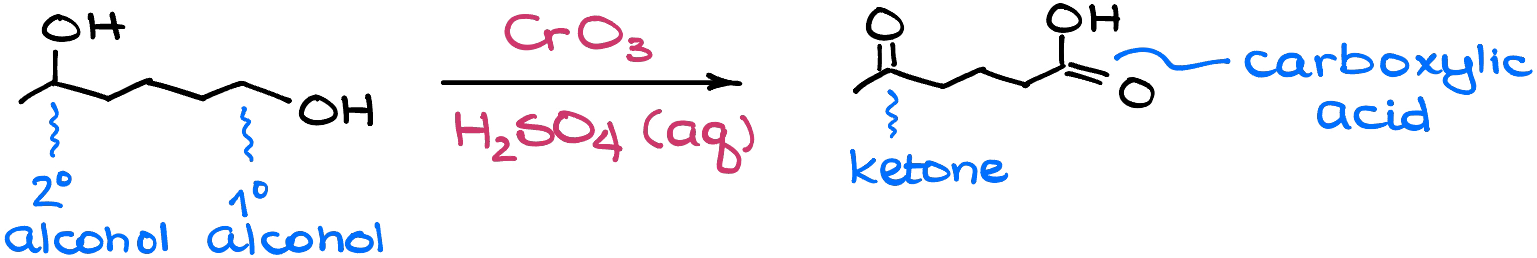
Aldehydes are not safe in this reaction either. Since aldehydes are actually intermediates in the Jones oxidation, they will get oxidized to the corresponding carboxylic acids as well.
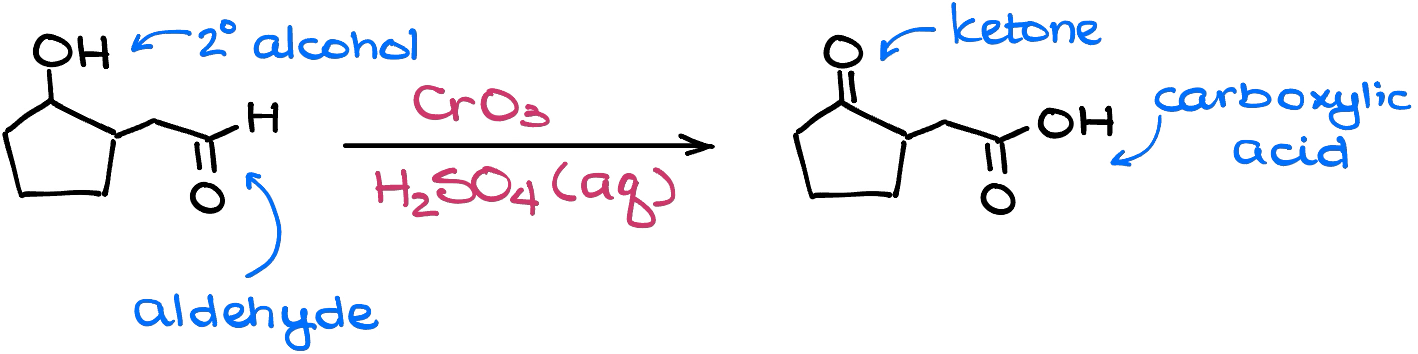
So, when you’re planning your synthesis, keep in mind the lack of the chemoselectivity of this reaction.
Hydrolysis of Nitriles
The next method is going to add an extra carbon to our molecule. In this method we’re going to incorporate a nitrile into our molecule via a simple substitution reaction (SN2), and then hydrolyze it.
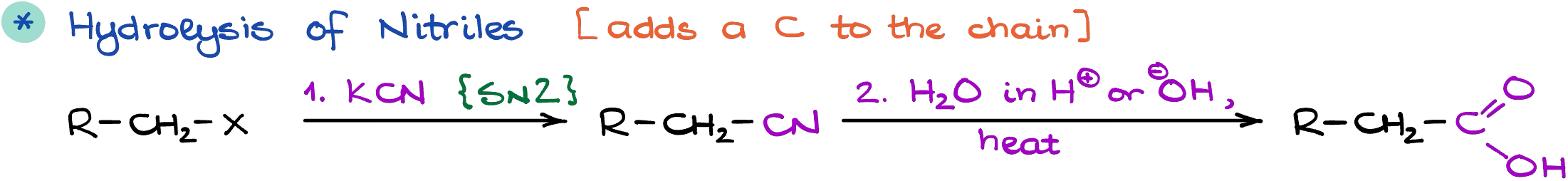
While the hydrolysis can be done in both basic or acidic conditions, the acidic hydrolysis uses milder conditions than the basic hydrolysis. Another limitation of this method is in the SN2 portion where we add the nitrile to our molecule. Since SN2 reactions are very sensitive to the steric hindrances, this method is not suitable for making highly substituted carboxylic acids in the ⍺-position.
The Grignard Reaction
A more universal way of the carboxylic acid synthesis is via a Grignard reaction.
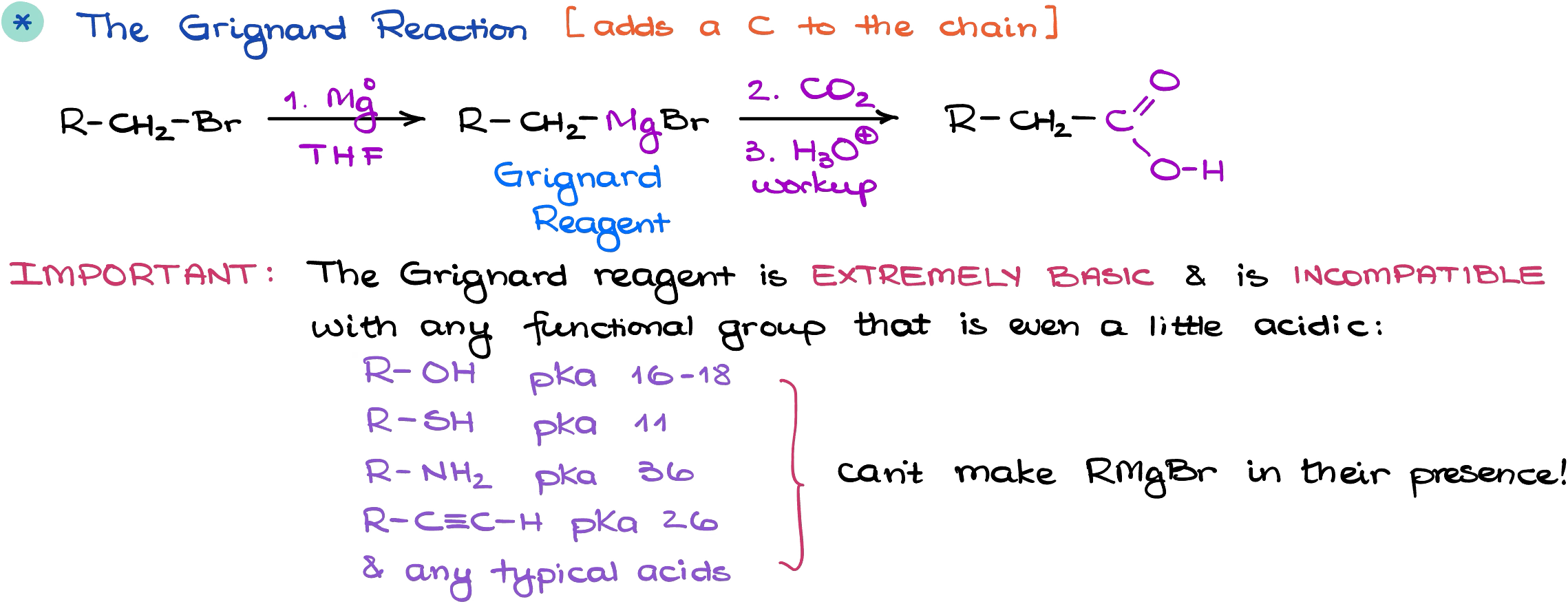
Alkyl halides are relatively easy to make and the Grignard reaction doesn’t have many limitations. For as long as we’re using alkyl halides that don’t have any acidic functional groups such as alcohols, thiols, or using acidic conditions, we can use virtually any alkyl halide to make the Grignard reagent.
Like in the previous case, we’re adding an additional carbon to our chain using this method. So, you gotta pay close attention to your molecular structure when planning your synthesis.
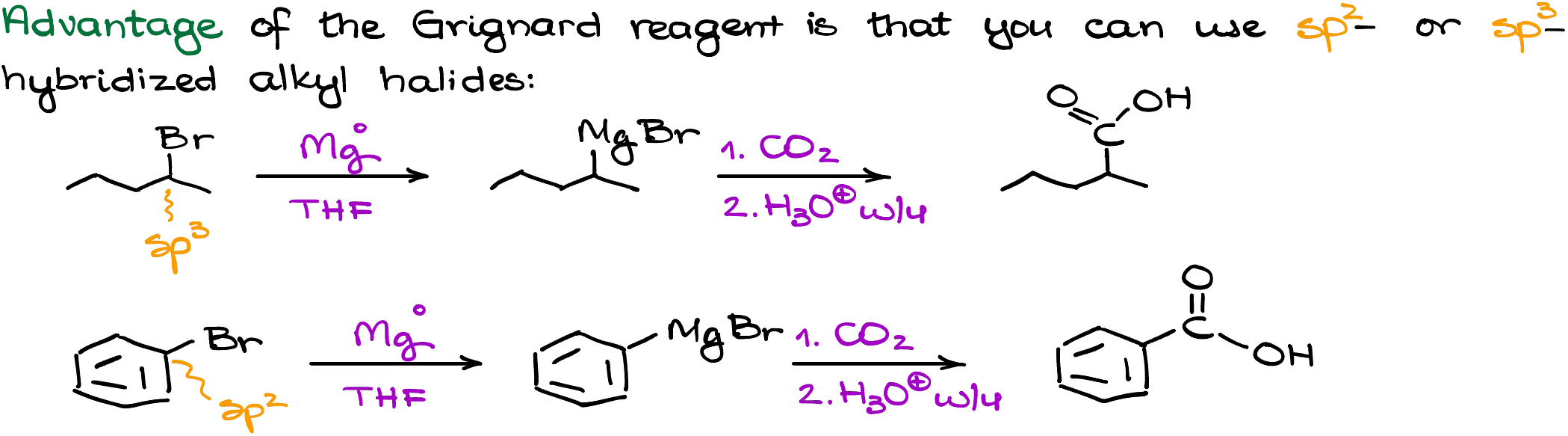
A huge advantage of the Grignard reaction is that you’re not limited by the nature of the alkyl halide. So, we can make carboxylic acids using the Grignard reaction that cannot be made using other synthetic approaches.
Ozonolysis of Alkenes and Alkynes
Finally, the last method of carboxylic acid synthesis is the oxidative cleavage of either alkenes or alkynes using ozone.

The important aspect of this reaction is that now we are cleaving some carbons from our molecule. So, your chain is going to end up being shorter. This is not particularly carbon efficient. And because of that, we tend to avoid this synthetic procedure. However, sometimes instructors like to add it to the multistep synthesis questions for a creative spin on the question.
Ozonolysis can, however, be a very useful synthetic approach when we need to make dicarboxylic acids via cleavage of a cyclic alkene.
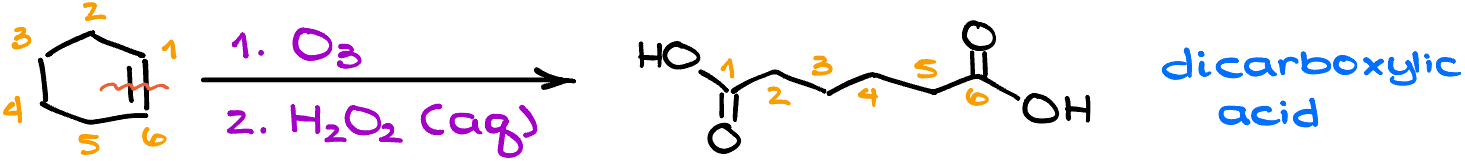
So, when your starting material is a cycle and you end up with some sort of an open chain molecule, you’re most likely going to be using the ozonolysis as one of the key steps in your synthesis.
Concluding Thoughts
As I’ve mentioned at the beginning, this list is far from exhaustive. However, these reactions are the typical ones that you can expect to use in your multistep synthesis on your organic chemistry tests or your homework assignments.
Practice Questions
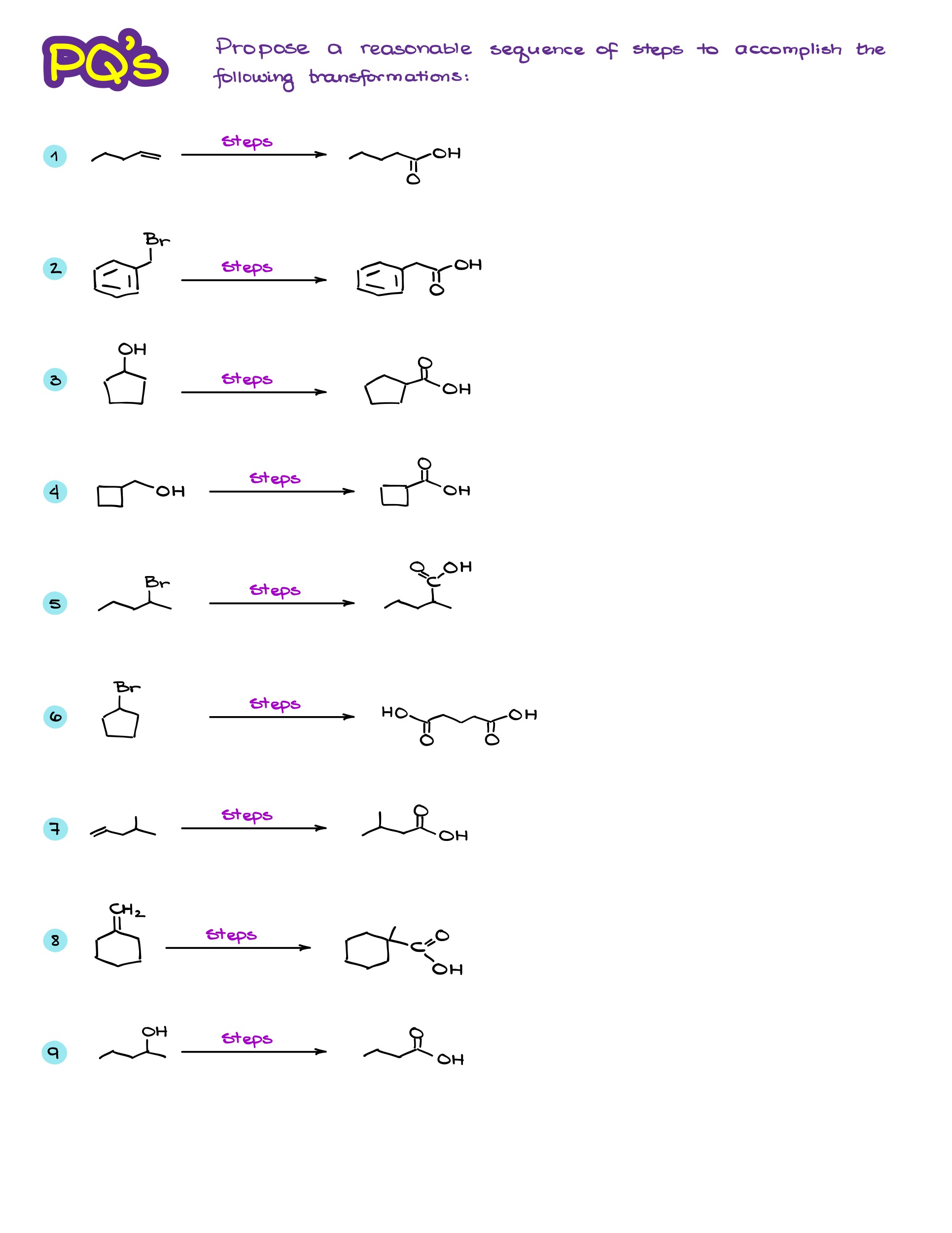
If you would like to check your answers, become a member or log in.
