Formation of Imines and Enamines
When amines react with aldehydes or ketones, they make either imines or enamines depending on the structure of the amine. Primary amines make imines — compounds with C=N bond.
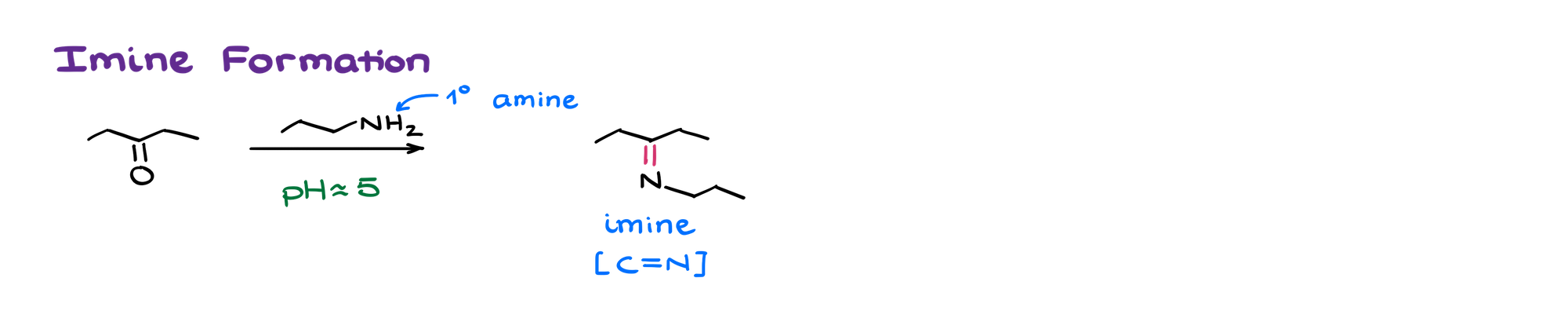
Secondary amines, when they react with aldehydes and ketones, make corresponding enamines.
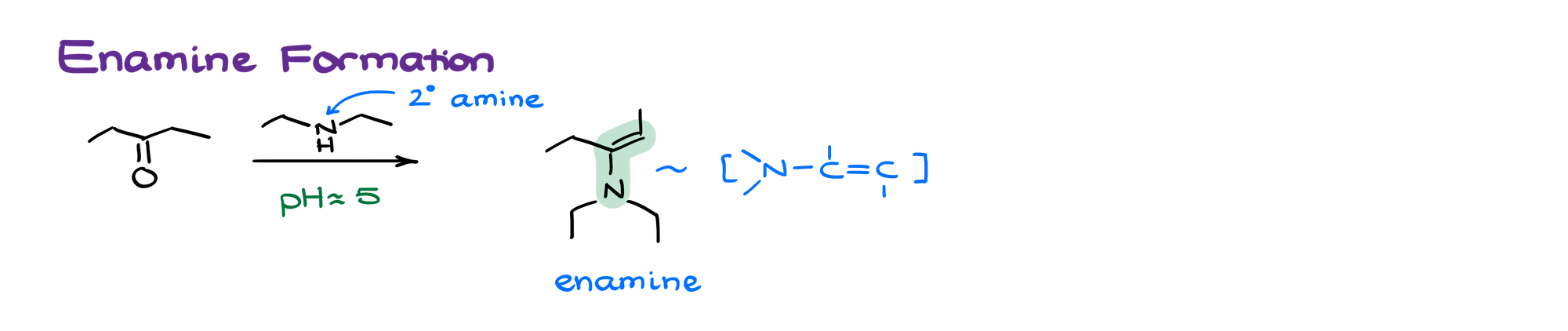
Enamines have a C=C bond adjacent to the nitrogen atom, rather than directly on it.
Both functional groups are not particularly interesting on their own. However, both have important uses in organic chemistry. Imines are typical intermediates in reductive amination (synthesis of amines). And enamines are particularly useful as stable alternatives for enolates in various C-C bond formation reactions. Specifically, enamines are used in the Stork enamine synthesis.
Depending on your course structure, you might be covering the formation of imines and enamines in the carbonyls chapter, or you might be seeing them in the amines chapter. Regardless when you’re going to be talking about those in your course, imines and enamines are a must-know topic in the sophomore organic chemistry, so you would need to know it for the finals for sure.
Mechanism of the Imine Formation
Both reactions have a similarly looking mechanisms.
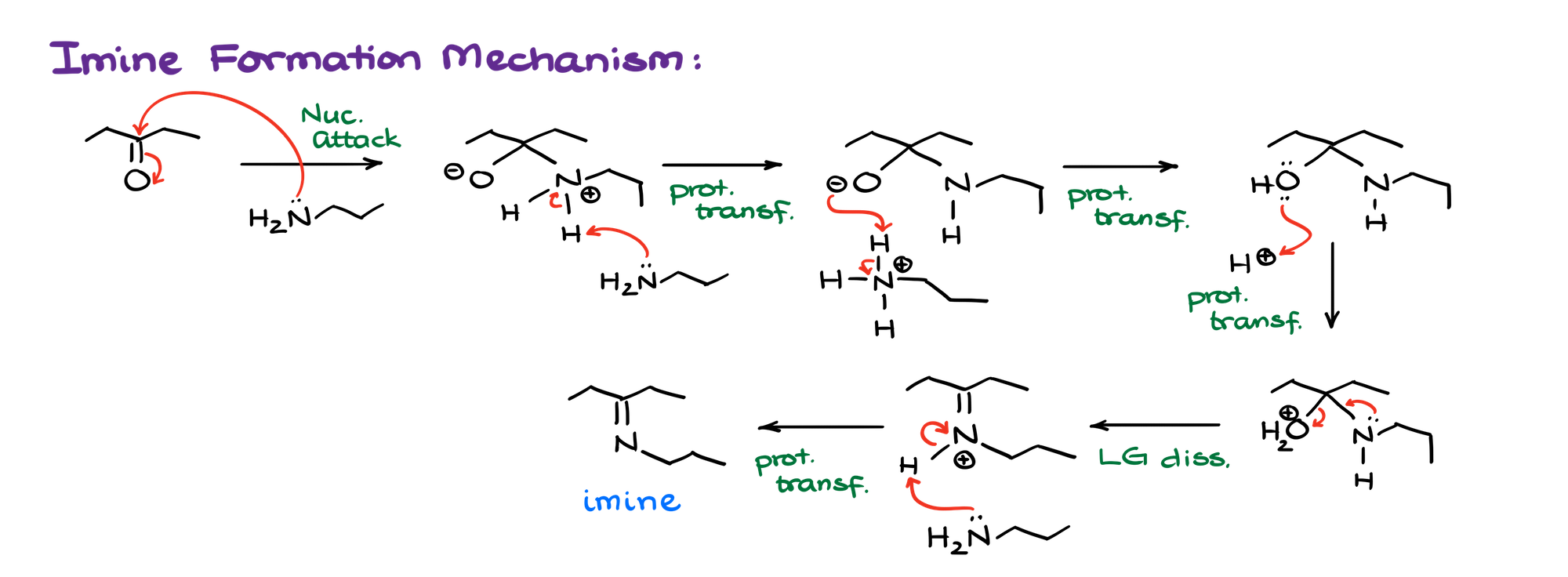
The reaction is catalyzed by a very small quantity of an acid. So, typically, we are going to show it as a pH=5. A very small amount of acid here is quite strategic. Any larger quantity would make your amine non-nucleophilic since the nitrogen of the amine will be protonated. Any smaller quantity — and your reaction will be rather slow and inefficient.
Mechanism of the Enamine Formation
Mechanistically speaking, the enamine formation is exactly the same except for the very last step.
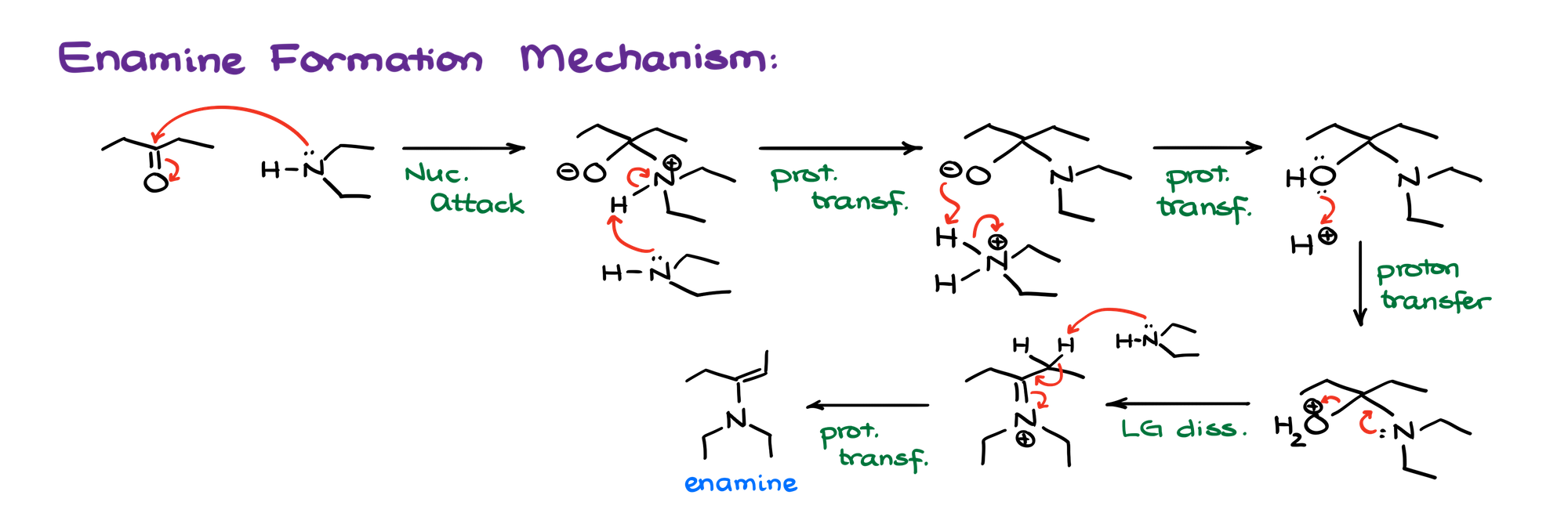
In the imine formation, the last step is a proton transfer that allows us to deprotonate an iminium ion and make a neutral final product. In the enamine formation, this is going to be impossible because we simply don’t have a proton on our nitrogen. So, we’ll have to remove a proton from an adjacent carbon instead.
Always Make a More Stable Product
Another thing to keep in mind about these reactions is that the formation of imines and enamines is an equilibrium. This means that we’re going to typically go for the more thermodynamically stable product in each case. This is especially important in the case of the non-symmetrical starting materials that can give you multiple stereoisomers.
For instance, let’s look at this reaction:
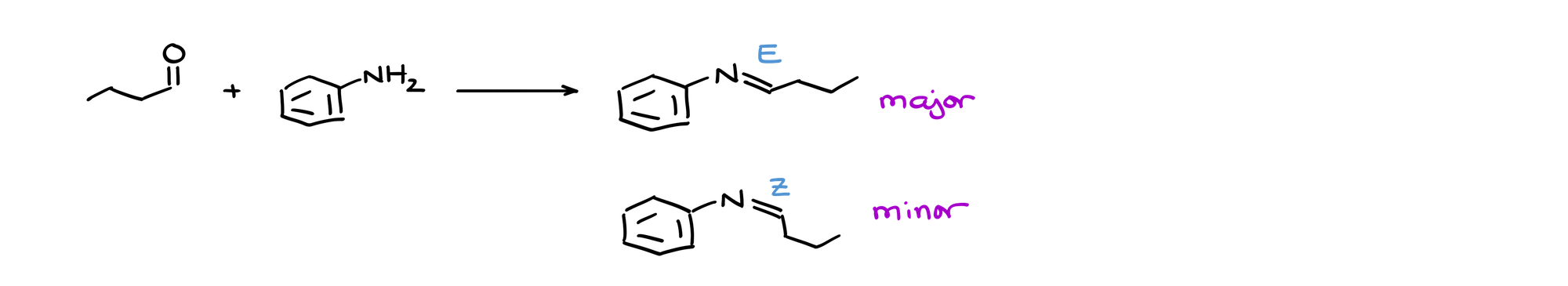
Just like any other double bond, the C=N double bond is rigid and therefore may form two possible stereoisomers. So, when you’re making your imines, opt for the product that would have the bulkiest groups trans to each other.
In the case of enamines, we have a similar situation:
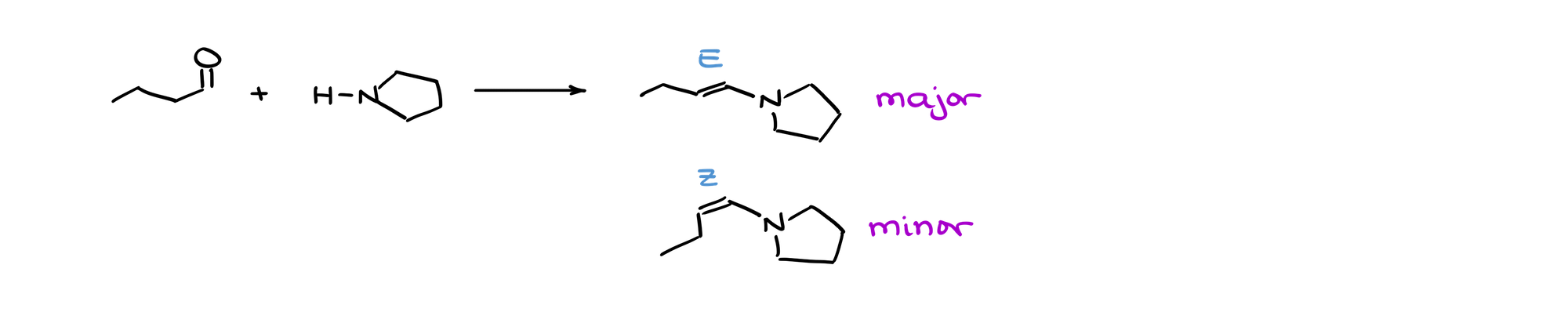
Like in the formation of imines, always opt for the more thermodynamically stable enamine.
So, as you can see, the formation of imines and enamines is a very straightforward reaction. And as I’ve mentioned earlier, it’s rarely going to be the final product but rather an intermediate in a multistep synthesis.
