Nomenclature of Aldehydes and Ketones
In this tutorial I wanna talk about the nomenclature of aldehydes and ketones.
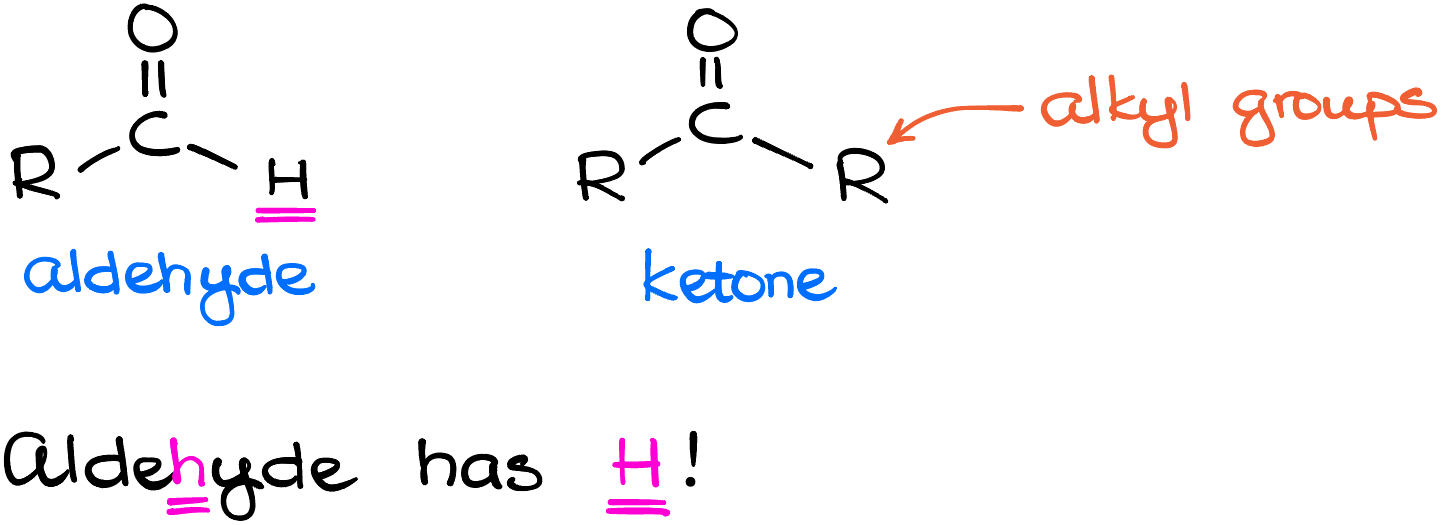
First of all, let’s talk about the difference between the aldehydes and ketones. Both functional groups contain the carbonyl, which is the carbon-oxygen double bond. Ketones have two other alkyl groups sitting on the carbonyls. Aldehydes, however, have only one alkyl group on them and the other group is a hydrogen. If you like, you can always use a mnemonic device to remember this: aldeHyde has an H. And while these two functional groups look very similar to each other, they are in fact different. Aldehydes exhibit reactivity that’s not accessible to ketones, so that by definition makes them different functional groups.
Naming Ketones
Let’s start by looking at ketones. When naming ketones, we’re going to use the ending “-one” which we’ll add to the end of our molecule. For instance, this molecule is going to be propanone, or acetone if you like to know the common name for it which you’ll see somewhat often in your course.
Now, this is a very simple example. What if we have something more complex.
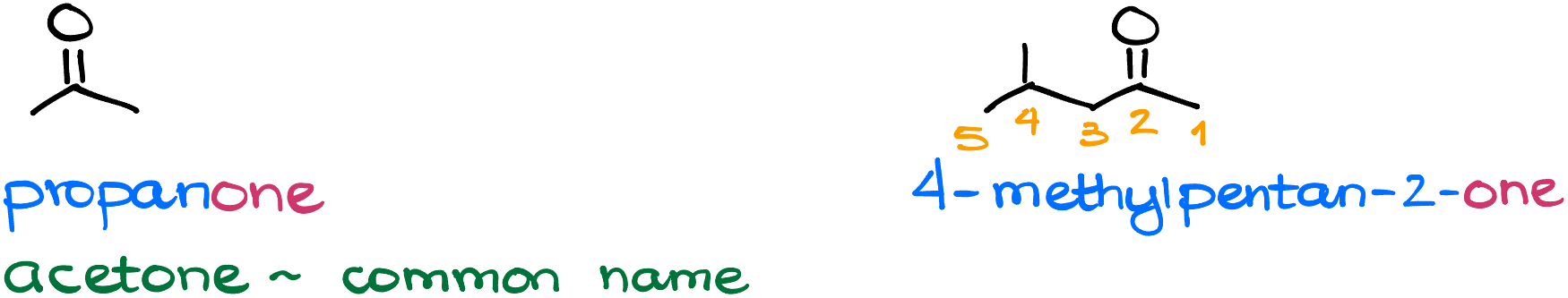
In this case we have a carbonyl and we also have a substituent (a methyl group). Since our carbonyl is a functional group, we are going to make sure we number the molecule in such a way as to give it the lowest possible number. Which means, in this case we’ll number our molecule from right to left.
Another thing to keep in mind, is that like in the case with all other functional groups, we’ll need to specify where exactly it is in the molecule. So, when we put the name together, we’ll say that this is 4-methylpentan-2-one. Here, the locant “2” specifies the position of the carbon with the carbonyl.
Naming Aldehydes
When it comes to aldehydes, the ending that we’re going to be using is “-al.” So, a two-carbon aldehyde would be ethanal. It’s also known as acetaldehyde by its common name. Another common name you might wanna remember is formaldehyde, which is the simplest aldehyde. The IUPAC name for it is going to be methanal but hardly anyone ever calls it this way.
Just like with the ketones, we’ll have to number our molecule to give the lowest possible number to the carbonyl of the aldehyde. But here’s a catch—the aldehyde is always going to be at the end of the chain. So, this means that you don’t even need to say that it’s at the position #1. This will always be assumed.

Thus, this molecule is going to be called 2-ethyl-3-methylpentanal. Keep in mind, that since the aldehyde is a functional group, you have to find the longest continuous chain with it even if this would give you a shorter chain overall. Like in this molecule, technically, the longest chain is 6 carbons long. But that chain doesn’t have the aldehyde, so it doesn’t count.
Another common mistake I see a lot of students make is not counting the carbon of the aldehyde functional group as a part of the chain. The carbon of our aldehyde is going to be the carbon #1. Always remember that and don’t just discard that.
Aldehydes vs Ketones
How are we going to deal with a molecule where we have an aldehyde and a ketone functional group? Quite easy, actually. Aldehydes have higher priority. So, when you’re numbering your molecule, you’ll have to start with the aldehyde regardless. Also, the ketone in this case will be noted using the prefix form rather than the ending form. The prefix form for carbonyls is “oxo” and it’s going to follow all the same rules as any other substituent which you might have in your molecule.
For instance, these two molecules are: 4-oxopentanal on the left, and 2-ethyl-4-methyl-3-oxopentanal on the right.
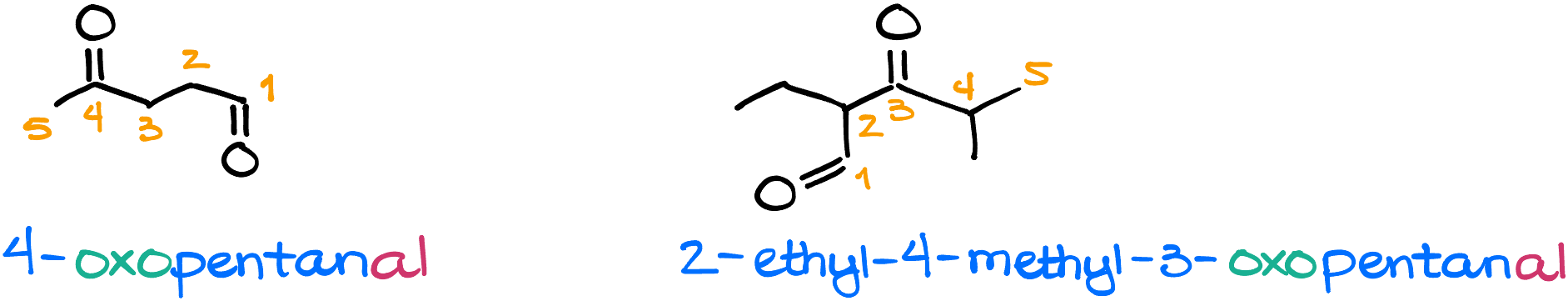
In terms of priority, aldehydes and ketones are above alcohols and pi-bonds. So, if your molecule has all of the above, you’ll still have to prioritize the aldehyde or a ketone for the purposes of numbering.
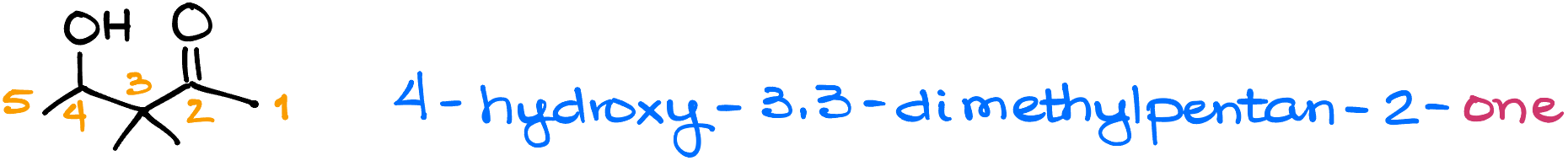
For instance, here I have 4-hydroxy-3,3-dimethylpentan-2-one. Here, I’ve prioritized the carbonyl over the alcohol in my naming.
Naming Cyclic Aldehydes and Ketones
There’s a huge difference in how we’re going to be approaching the nomenclature of the cyclic aldehydes and ketones. With ketones, it’s pretty straightforward. We number the cycle from the carbon where the ketone is located to give the lowest possible locants to the rest of our substituents or functional groups, alphabetize the substituents, and put it all together.
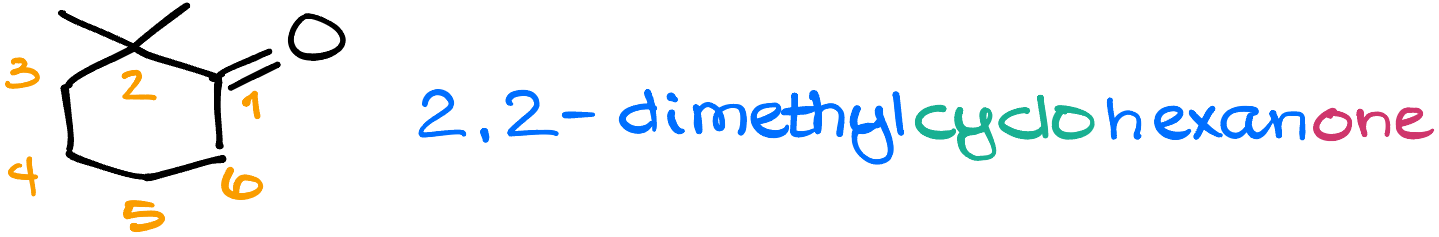
For instance, here I have 2,2-dimethylcyclohexanone. Nothing unexpected.
With aldehydes, however, it’s different. The thing is, it’s impossible to have an aldehyde directly be a part of the ring. It can only sit on the ring. And if we do not have any additional carbons between the aldehyde functional group and the ring itself, we can use a special style of nomenclature where we use “-carbaldehyde” ending.

So, this molecule would be called cyclohexanecarbaldehyde.
And that’s all you gotta know about the nomenclature of aldehydes and ketones. Of course, when it comes to organic chemistry, practice makes perfect, so make sure you practice nomenclature on a regular basis so you don’t get caught off-guard during the test as some instructors love testing for nomenclature.
