Basicity of Amines
In this tutorial we’re going to go over the fundamentals of the amine basicity. Normally, when we’re talking about the acid-base properties of molecules, we take a molecule, rip the acidic proton off that molecule, and analyze the resulting conjugate base. This allows us to determine which compound is more acidic or find the most acidic proton in the molecule. If you need a bit of a refresher on this topic, I have a ton of tutorial on the acid-base equilibrium. In the case of amines though, the amine itself is a base. So, we can either analyze the amine itself or look at the resulting conjugate acid. But let’s take it one step at a time.
Acid-Base Equilibrium with Amines
Let’s look at a simple acid base reaction, where I have an amine as a base (butyl amine here) reacting with an acid, for instance, HCl. Here, amine is going to be accepting the proton from my acid and forming the corresponding conjugate acid—protonated amine. This is, of course, a very simple reaction, I would fully expect you to be able to write it on your own. The trickery comes to the scene when we start dressing up our amine with different functional groups around it which can drastically alter its basicity.

What is Basicity?
And before we proceed, I wanna review the term “basicity” to make sure we are talking about the same thing here. Basicity refers to the molecule’s ability to accept protons from the environment. And the more basic the species is, the better it is at grabbing those protons.
So, just like in the case of the acidity, we can express basicity with a certain number. For acids, we used the pKa values. For bases we can use the pKb values. And we remember from general chemistry, that pKa + pKb = 14. Nice, easy, and convenient, right? Well, actually no. It is easy, yes, but convenient? I wouldn’t go that far! The thing is, this relationship between the pKa and pKb values only applies to aqueous solutions. And how often are we dealing with the aqueous solutions in organic chemistry? Next to never. So, what are we going to do?
The easy solution is to use the pKa values. But we’re not going to be looking at the pKa values of the amines themselves, rather, we’ll be looking at the pKa values of the corresponding conjugate acids! And we’ll be referring to those as pKaH values.
For instance, the typical pKa values of protonated amines ranges between 9 and 10. However, depending on the nearby groups, it can drop as low as 4 or 5, which is already comparable to carboxylic acids in strength!
Factors Affecting Basicity of Amines
So, what factors affect the basicity of amines? If a base is the proton acceptor, then anything that will increase the electron density on the nitrogen will make it more basic. While anything that decreases the electron density on the nitrogen will make it less basic.
Resonance
The first factor I wanna talk about is the resonance. Now, I’m not going to be looking at the resonance in the molecule in general. I’m only interested in the resonance that involves the electron pairs on the nitrogen atom. For instance, if I look at the cyclohexanamine vs aniline, the former’s electrons on the nitrogen are localized, while the latter one’s nitrogen electrons are delocalized through the aromatic ring. If you feel a little rusty on your resonance, make sure you review this topic, coz it’s gonna be big for the basicity of different amines.
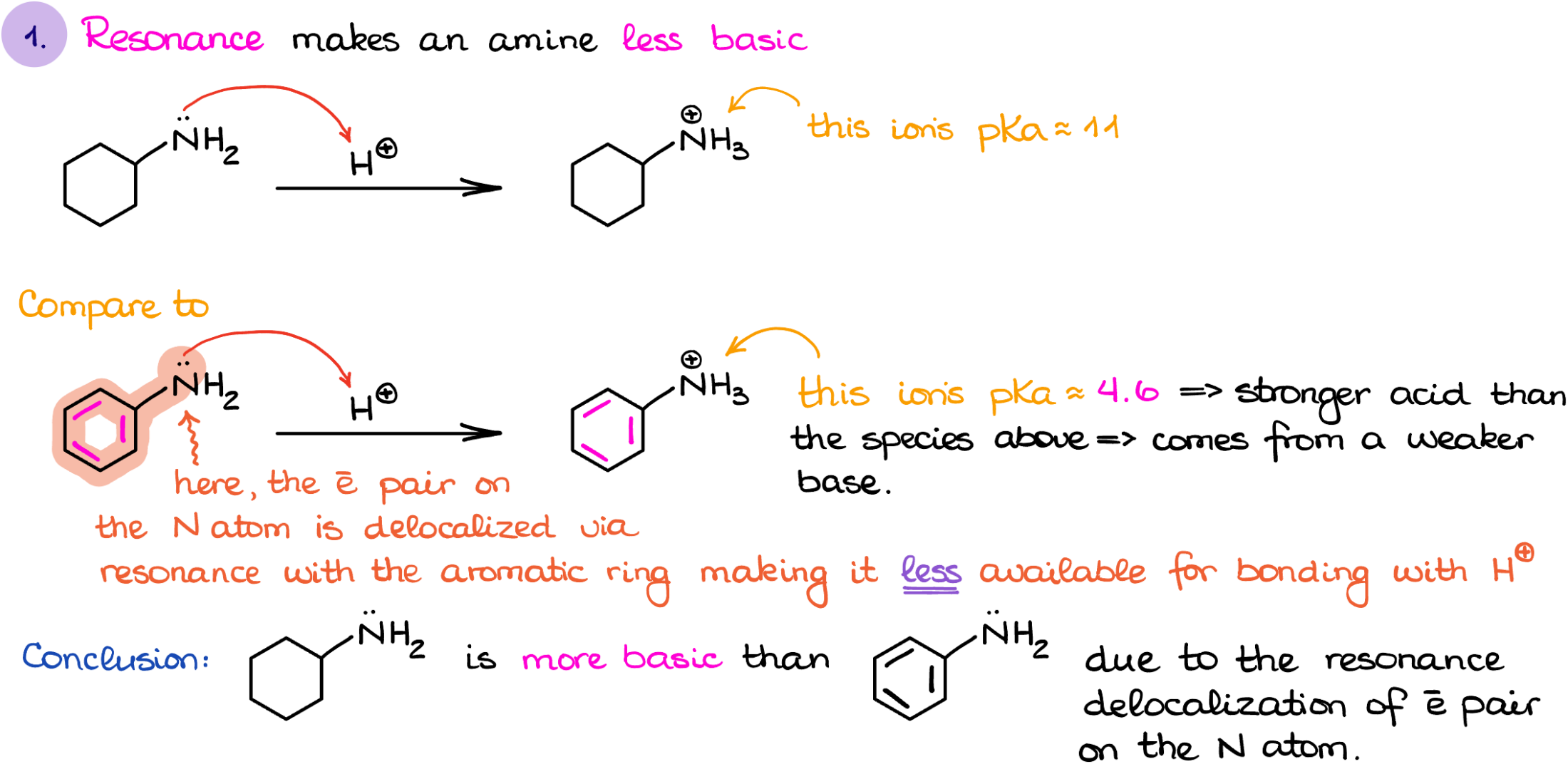
This way, if I were to protonate each of those molecule and look at the corresponding conjugate acids, I can compare the pKaH values for them and determine which one is more acidic. The protonated cyclohexanamine’s pKa value is about 11, while the protonated aniline is around 4.6. Which means that the cyclohexanamine is more basic than aniline.
Why such a drastic difference? Think about the electron pair on the nitrogen and its availability. If it is delocalized, it is already preoccupied doing something. This means that the nitrogen will be less likely to donate those electrons to make a bond with a proton.
Induction
The next factor is the induction. While induction is not as strong of a factor as resonance, it can be quite significant depending on the structure of the amine and the distance to the electron-withdrawing group.
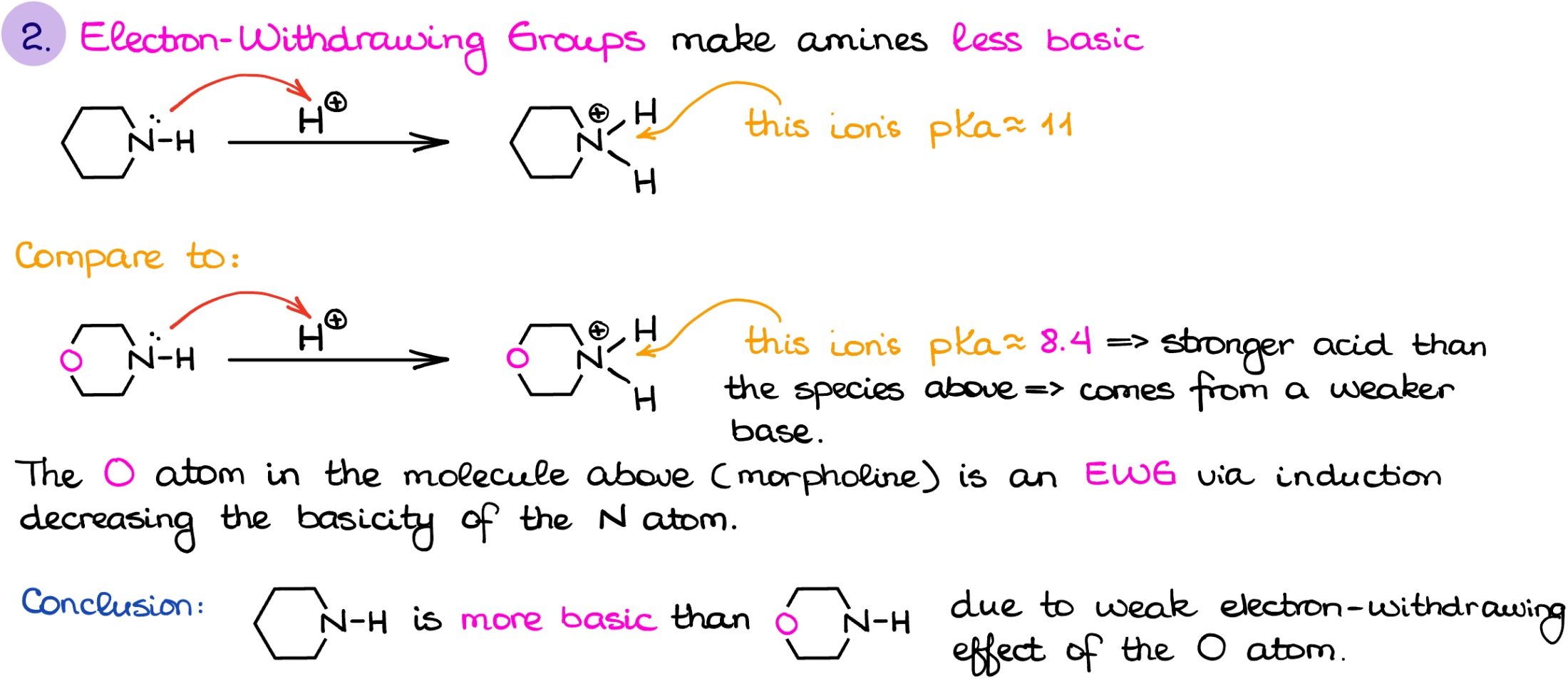
For instance, here I have an example of piperidine and morpholine. If I protonate each and compare the pKa values of the corresponding conjugate acids, we’ll get 11 for piperidine and 8.4 for morpholine. While the difference is not as drastic as in the last case, it is nonetheless there. And this difference is due to the electron-withdrawing, or in other words inductive, effect of the oxygen atom.
Hybridization
Hybridization is another factor that we need to keep in mind when we’re talking about the basicity of amines. The lower the p-character of the orbital with the electron pair, the less basic it is going to be.
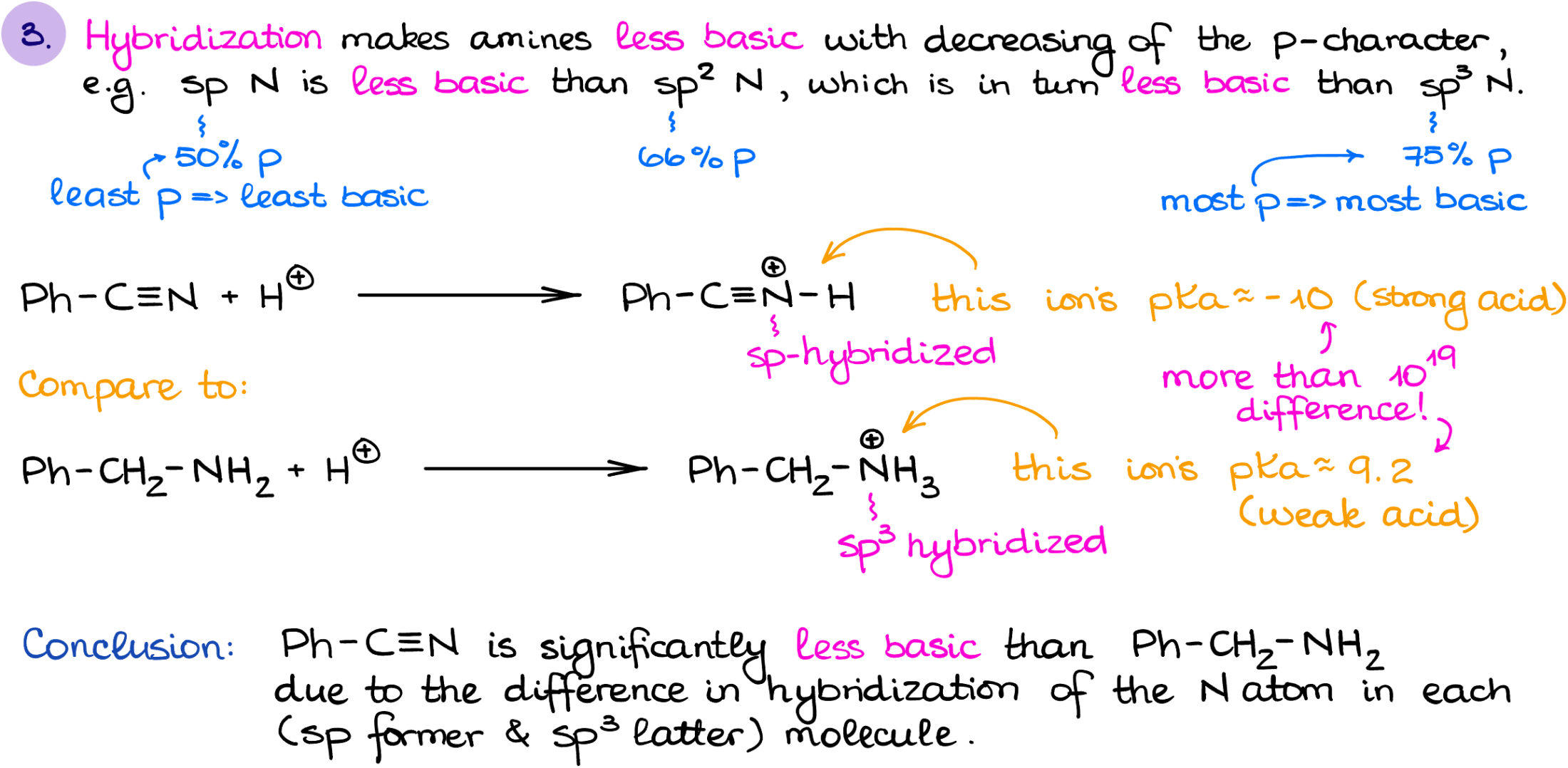
So, for instance, if I were to compare the basicity of a nitrile vs the primary amine like in this case, we’ll see that the nitrile is significantly less basic than a primary amine. Now, I wanna mention that the hybridization is a treacherous factor. I suggest you look at other factors first and see if you can find any differences before you jump into the hybridization considerations.
Steric Hindrance
Steric hindrance is the last factor you should keep in mind when thinking about the basicity of amines. While we generally consider alkyl groups as electron-donating species which should increase the electron density on the nitrogen, don’t forget that those guys do occupy some space. And the less space you have around the nitrogen atom, the more difficult it would be for that nitrogen atom to grab the proton from another molecule.
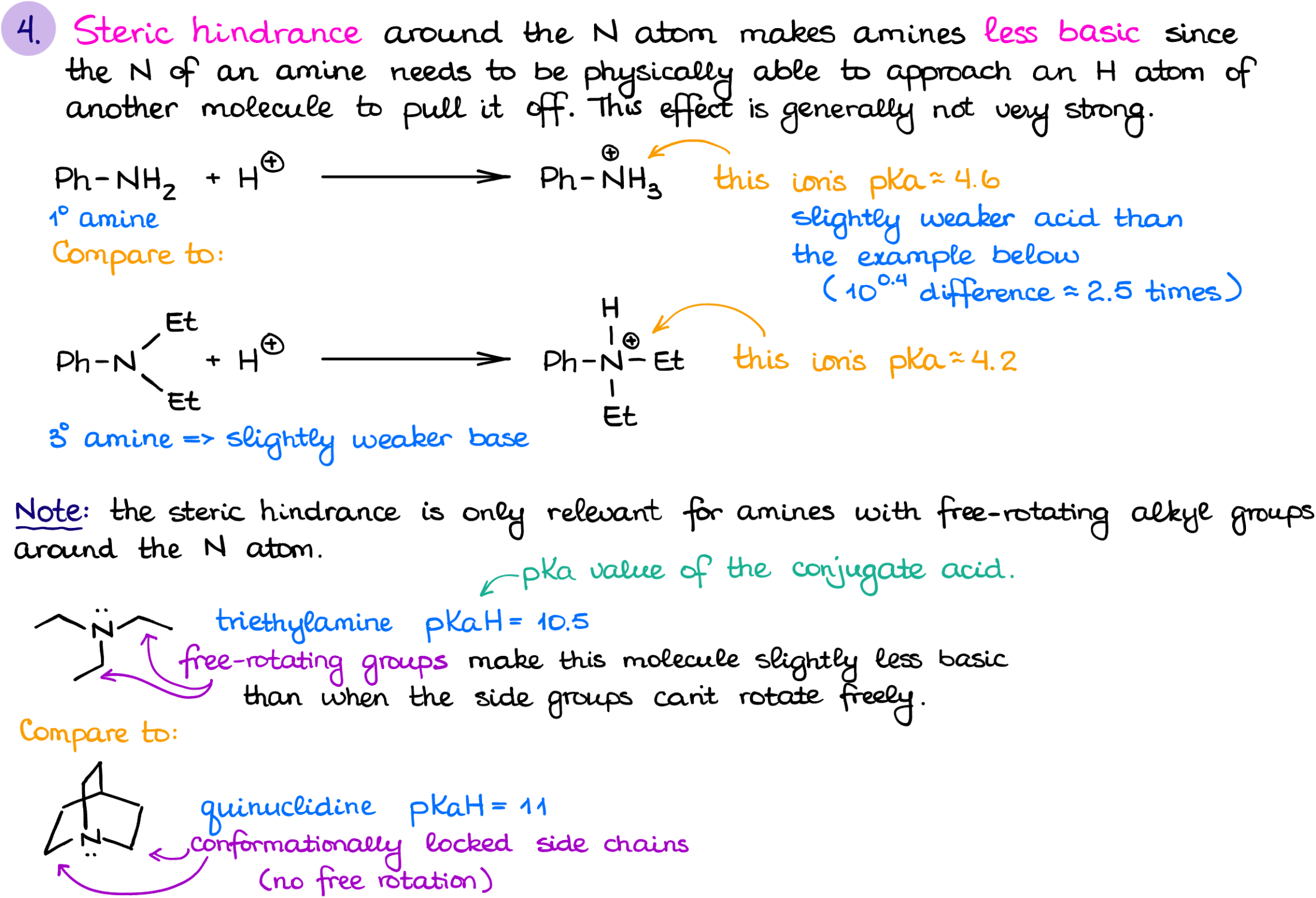
So, generally, tertiary amines are somewhat less basic than secondary amines. The difference is typically not too drastic, but some instructors like to test for it, so you should know this. The only time when a tertiary amine would be more basic than a similarly looking secondary one, is when the molecule is conformationally locked and the alkyl groups can’t make too much of a mess around the nitrogen. An examples of such molecules would be quinuclidine or DABCO. Since we do not have a free rotation around the single bonds in those molecules, the alkyl groups will provide the positive electronic effect and won’t be able to cause much of steric hindrance making those amines abnormally basic for their structure.
Concluding Thoughts
Of course, a quick tutorial cannot give you the answers for all possible variations that you might face on the test. However, these factors will always be your first steps in your amine analysis. So, make sure you do plenty of practice to build up your detective skills in finding all the factors that may influence the amine basicity.
