Epoxide Opening
Opening of epoxides is a seemingly simple reaction that can catch you by surprise if you’re not paying attention to the conditions and the reagents you’re working with. In this tutorial on epoxide opening reactions, we’ll focus on the regioselective opening of epoxides under acidic and basic conditions. Epoxides, known for their reactive three-membered ring structures, demonstrate distinct behaviors depending on the environment, leading to varied outcomes in synthesis. We’ll delve into the nuances of these reactions, highlighting the differences between opening epoxides from more or less substituted sides, and uncover the principles behind these pivotal organic reactions.
Epoxide Opening Overview
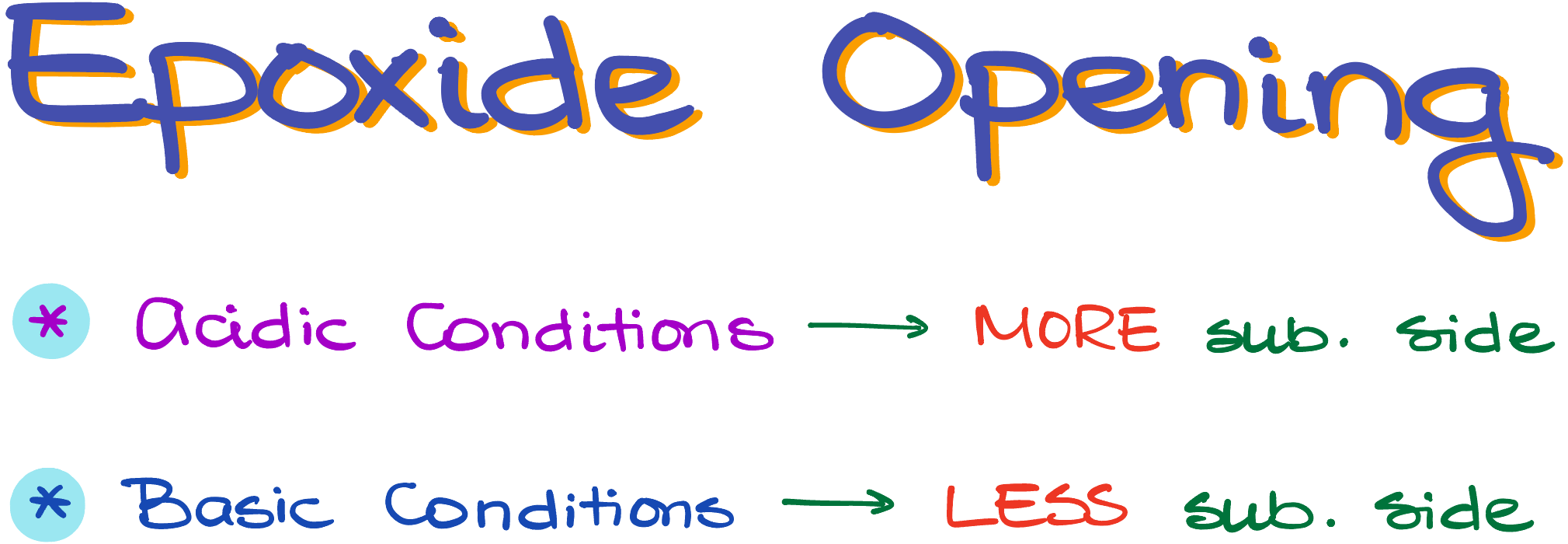
As I’ve mentioned a moment ago, epoxides can be opened in both acidic and basic conditions. The main difference between the two approaches is going to be the regioselectivity of the reaction. And by the regioselectivity here I mean which side we’re going to open our epoxide ring from.
So, if we look at an epoxide, it’s a three-membered ring with an oxygen atom in the ring. We also call epoxides oxidants, which is the IUPAC name for this functional group. If the molecule is not symmetrical, we can distinguish between the more and less substituted sides of the ring.
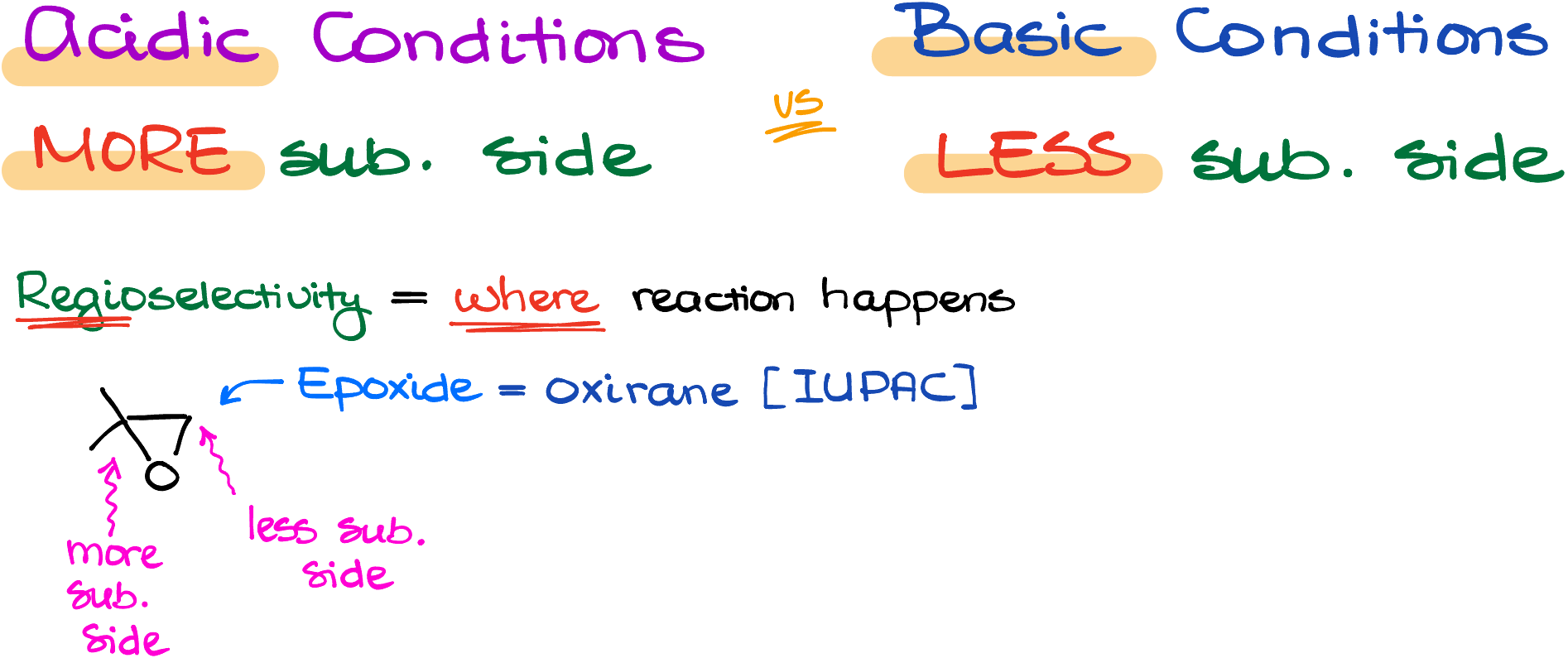
Now, depending on the nature of our reagents and conditions, the ring can open from either the more substituted side or from the less substituted side. TLDR; version is the acidic conditions open epoxides from the more substituted side, while the basic conditions open the epoxides from the less substituted side.
Now, let’s talk about the details of why that happens and how.
Epoxide Opening in Acidic Media
I’ll start by looking at the epoxide opening in acidic media. In acidic condition we’ll have a strong acid like H2SO4 present as a catalyst, or the reagent itself will be a strong acid, like, for instance HBr.
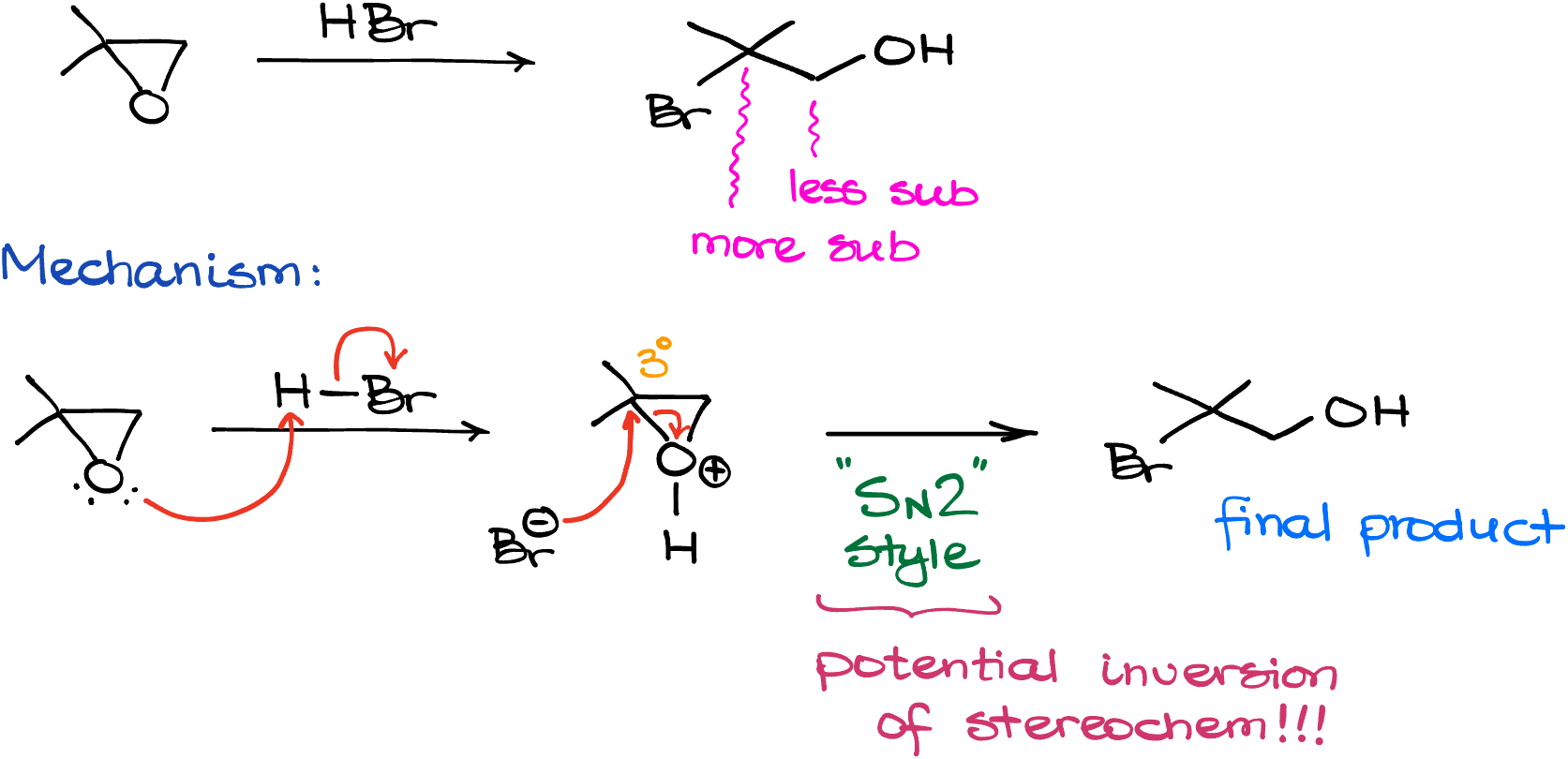
So, if I took my example molecule, 2,2-dimethyloxirane here, and treated it with HBr, I’d get the product, in which the oxygen will be still attached to the less substituted carbon of what used to be an epoxide ring, and the bromine will be connected to the more substituted carbon. Mechanistically speaking, this reaction starts by protonating the oxygen of our epoxide, followed by the ring opening by the nucleophilic bromide anion.
Interestingly enough, although this is a tertiary atom, the reaction seems to follow the SN2 kinetics. And, if any stereochemistry is present, we’ll see the inversion of stereochemistry here as well. Occasionally, we’ll see the formation of a carbocation in reactions like that, but those are not all too common. And even though there’s a possibility of a carbocation formation, we’ll treat this reaction as a “special case” of SN2 which works for tertiary carbons. We’ll also attribute this unusual reactivity to the ring strain.
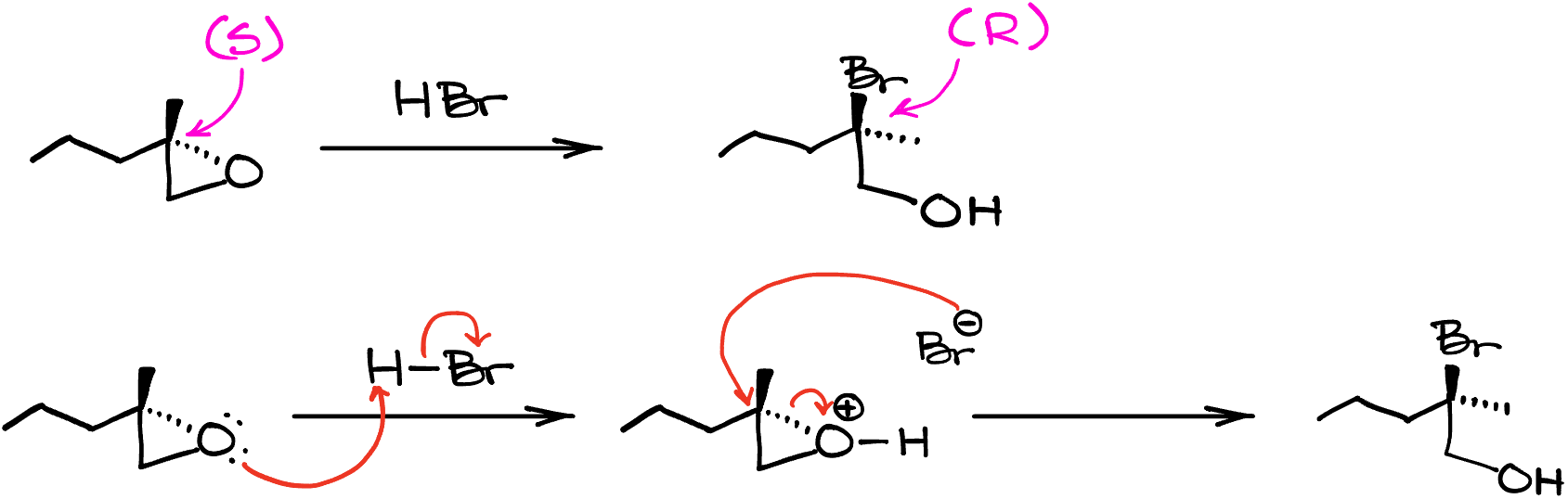
Now, I’ve mentioned that we’ll also see the inversion of stereochemistry here. Indeed, if I took this molecule and treated it with HBr, the resulting product would have a carbon with the opposite stereoconfiguration. You can confirm that we do see the inversion here by assigning the stereodescriptors to our chiral atoms. The molecule on the left is the (S)-stereoisomer, while the product has the (R) stereoconfiguration. And by the way, using this opportunity I’m going to remind you that the inversion of stereoconfiguration does not always coincide with the change in the stereo descriptor. It works in most cases, but it depends on the nature of the leaving group and the nucleophile. And occasionally, you’ll end up with the same stereodescriptor for both starting material and the product because the CIP priorities can get all switched around with the new group in the molecule.
Epoxide Opening with Neutral Nucleophiles with Acid Catalysts
What if my reagent is not a strong acid on its own? Well, in this case, we’ll need to bring acid as a catalyst. Since we don’t want any competition between our conjugate base and the nucleophile, we’ll use non-nucleophilic acids like H2SO4 for our catalyst.
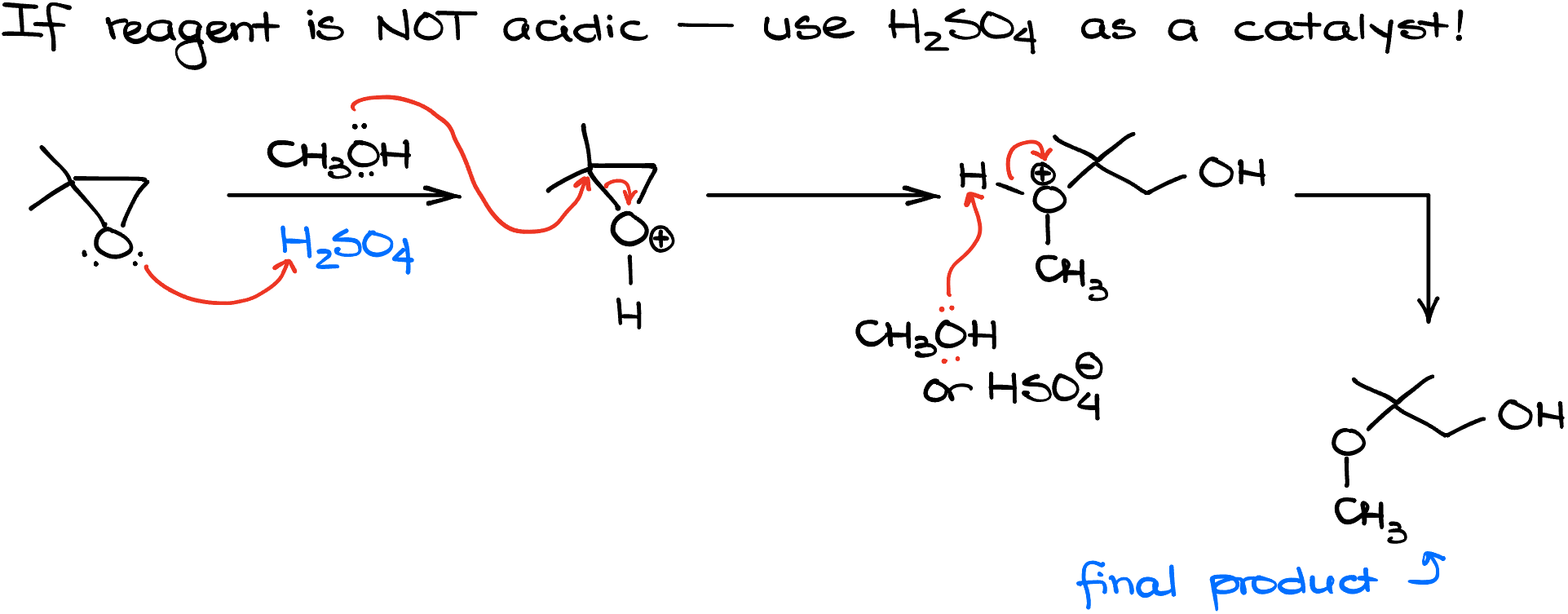
For instance, if I go back to my 2,2-dimethyloxirane and treat it with methanol, nothing will happen as methanol is not nearly acidic enough to protonate my epoxide and make it more reactive. This is where the sulfuric acid becomes important. It provides the acidic protons and enables this reaction to happen. So, we’ll protonate the epoxide first, and then a poor nucleophile that is our methanol can attack it. It happens from the more substituted side like in the previous examples, giving us the corresponding ether as the final product.
Epoxide Opening in Basic Conditions
Moving on to the opening of our epoxide in basic conditions, I wanna mention right the way, that it’s not always actually the basic conditions. I suppose, it’s called this way to drive the the point and emphasize attention on the differences in the conditions. And it’s easy to remember acid vs base. But it should’ve been called more like “epoxide opening with strong nucleophiles” instead. Yes, it doesn’t sound nearly as good, but it’s a more correct version. The thing is, while some nucleophiles are indeed bases, many are not.
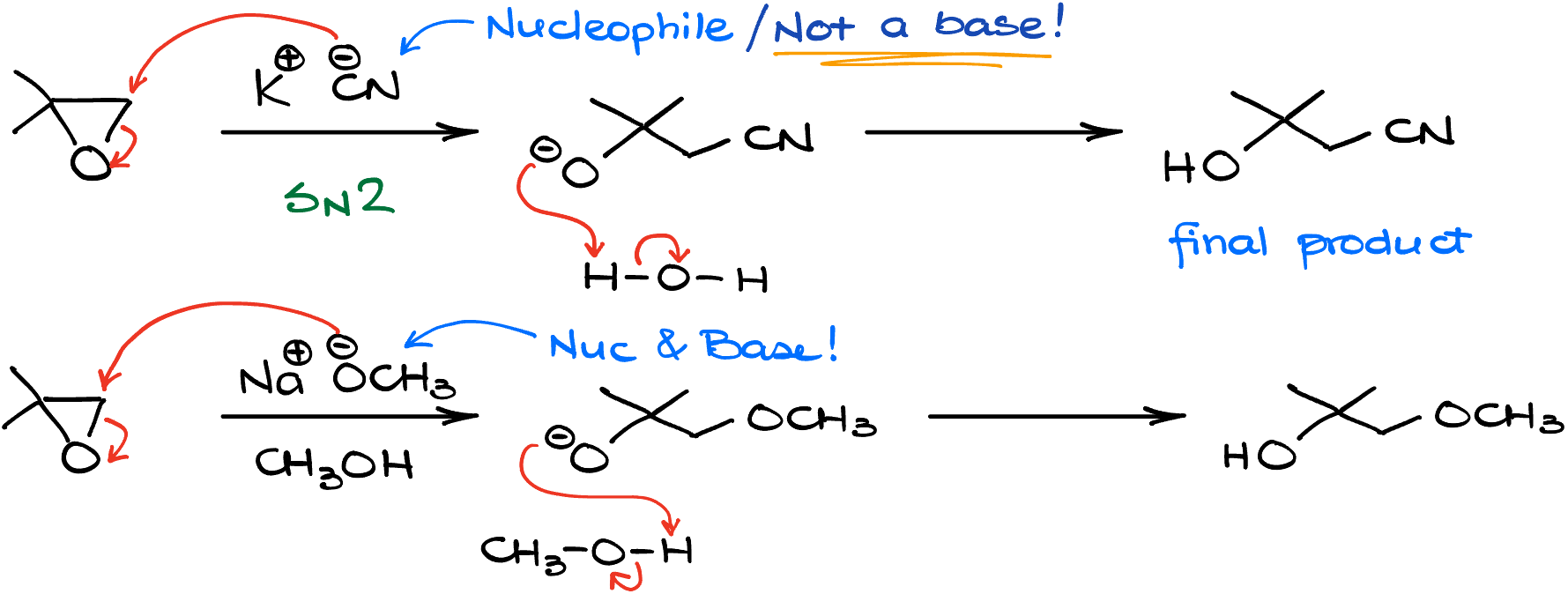
For instance, if I treat my 2,2-dimethyloxirane with potassium cyanide, the cyanide is indeed a nucleophile. Is it a base though? Not at all!
Another important distinction here is that the strong nucleophiles typically open our epoxides from the less substituted side. So, mechanistically speaking, the reaction is your typical SN2 process. The nucleophile, the cyanide anion in this case, attacks the carbon breaking the carbon-oxygen bond and making an alkoxide intermediate, which quickly grabs the proton from the solvent giving us the final product.
And yes, our nucleophile can be a base as well. Like for instance, if I were to treat our good friend 2,2-dimethyloxirane here with a solution of sodium methoxide in methanol, I’ll get the methoxide attack the epoxide from the less substituted side as well. And as we know from all our previous organic chemistry studies, alkoxides are fairly basic.
Does it matter that the methoxide a base though? Nope, not really, unless, of course, you have some other acidic functional groups in your molecule that can participate in a proton transfer reaction. What does matter though, is that methoxide is a good nucleophile. And since the world of nucleophiles is really diverse, we have a plethora of possible products that we can make by opening epoxides with different nucleophiles. And what’s really cool is that they all will open epoxides from the less substituted side making this reaction a powerful tool in our synthesis arsenal!
