Synthesis of Epoxides
In this tutorial, we’re delving into the world of epoxide synthesis, an important topic in organic chemistry. Epoxides, with their unique three-membered ring structure, play a pivotal role in various chemical reactions and are essential in both academic studies and industrial applications. We’ll explore the two primary methods for creating epoxides:
- the epoxidation of alkenes with peroxy acids, and
- the process of forming epoxides from halohydrins through intramolecular SN2 reactions.
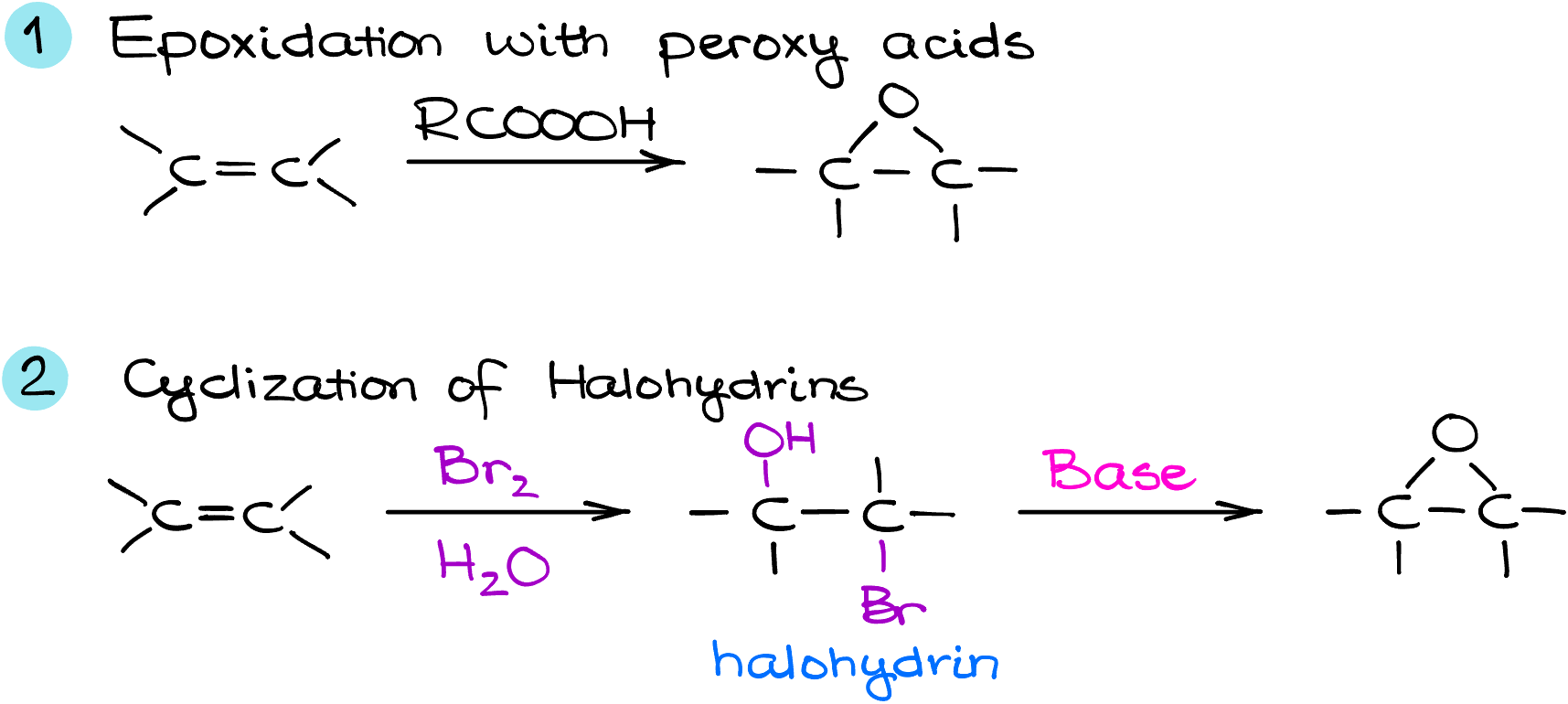
As we discuss these methods, we’ll talk about the nuances of each technique, emphasizing the importance of stereochemistry and the practical considerations that influence the choice between these pathways.
Epoxidation of Alkenes with Peroxy Acids
Epoxidation of alkenes is a fairly typical reaction of alkenes that you’ve already seen in your course. It’s also sometimes referred to as the Prilezhaev oxidation or Prilezhaev reaction. But on an off chance you didn’t see it or don’t remember it, let’s look at it and its mechanism.
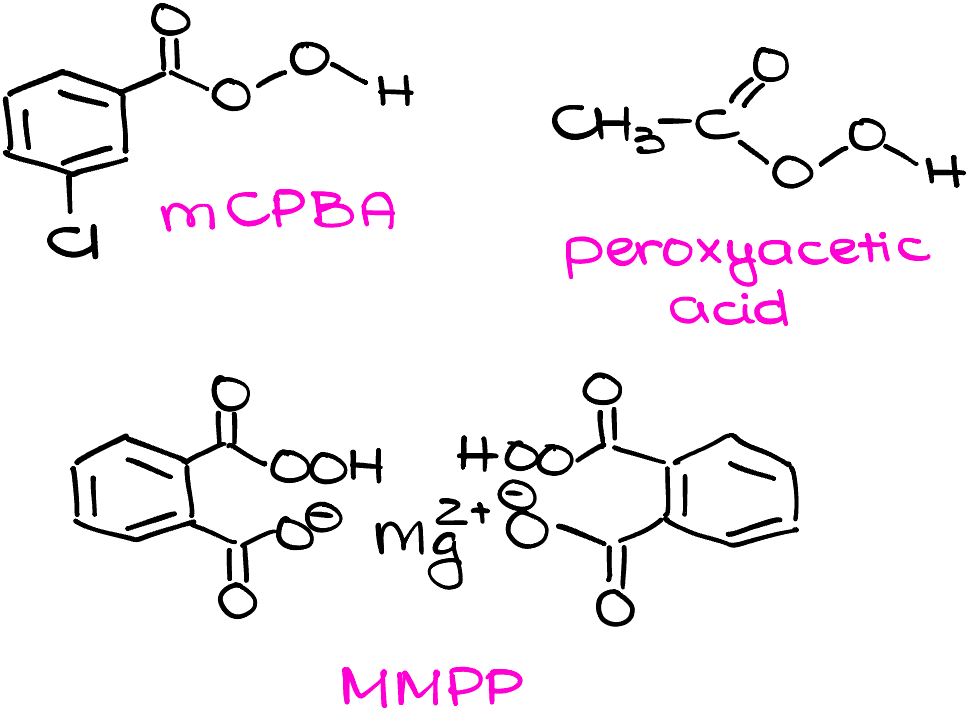
The most common epoxidation agent you’re going to see in your course is likely going to be mCPBA (meta-chloroperoxybenzoic acid). However, any other peroxy acid will work just as fine. You might also encounter peroxyacetic acid, and MMPP. For our purposes, those peroxy acids are interchangeable.
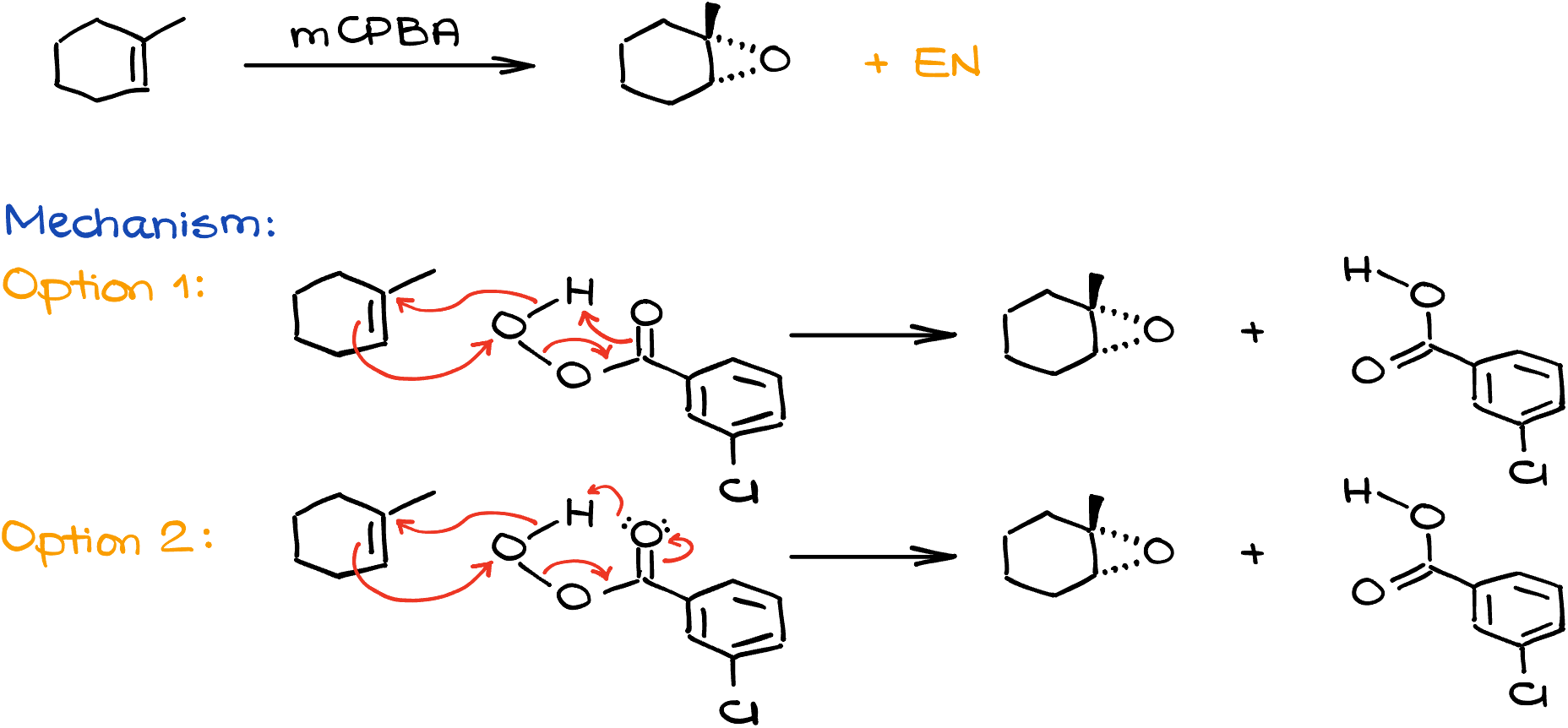
So, for instance, if I take 1-methylcyclohexene and treat it with mCPBA, I’ll end up with the corresponding epoxide. Mechanistically speaking, this entire reaction is a concerted process that makes an epoxide in one mechanistic step. There are two ways how we show this mechanism. One—with four arrows, another one—with five. Typically, instructors and textbooks prefer one or the other, so make sure you double check which one you’re using in your course. They both are correct for our purposes, so it’s not like one is strictly better than the other, it’s not.
I do wanna point out, however, that this reaction is stereospecific, so you gotta keep attention to how you draw your product based on how the starting material looks like. For instance, if I take the (E)-pent-2-ene and treat it with a peroxy acid, I’ll get a different product than if I were to do the same reactions with the (Z)-pent-2-ene.

How to Draw an Epoxide with the Correct Stereochemistry
And while we’re here talking about the stereochemistry of the epoxidation reaction, it might be a good idea to also quickly go over how you draw those pesky epoxides. If, for example, I took (E)-1-phenylprop-1-ene and treat it with the peroxy acid, like mCPBA, I’ll get the corresponding epoxide. However, since we normally zig-zag our bond-line structures, drawing this compound will be somewhat awkward keeping our skeleton intact. You might see your instructor and your textbook taking a slightly different approach to these drawings. Often, we’ll keep the epoxide in the plane of paper, while arrange the rest to the molecule around it.
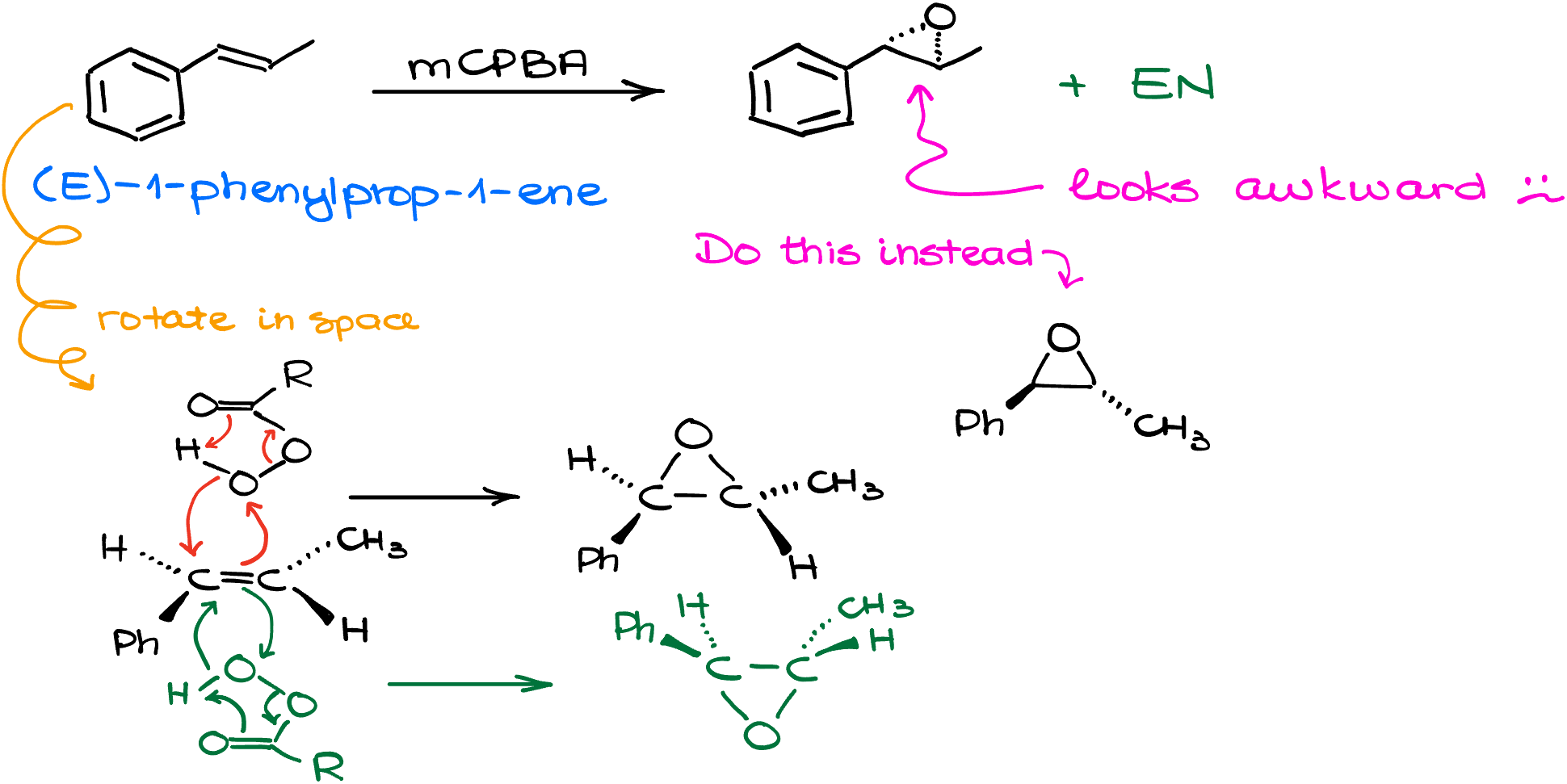
Let me illustrate it with an example. I’ll take the same starting material, but I’ll put this molecule on the side. So this way, the phenyl group will be looking at me, while the methyl group is looking away. Now, if I bring the peroxy acid from the top face, I’ll end up with the epoxide looking “up” in the plane of paper, while the rest of the groups are still looking in the same relative directions where they used to look before the reaction. Likewise, if we attack our alkene from the bottom face, we’ll end up with the epoxide looking “down” but still in the plane of paper. This way, you’re pointing the rest of the molecule in different directions rather than trying to poke the epoxide ring towards you or away from you. Drawing the epoxide on dashes and wedges while zig-zagging your molecule is not necessarily incorrect, but it’s awkward and can potentially be ambiguous. So, be careful with how you draw these molecules.
If it’s hard to imagine these molecules in 3D, you might wanna play with your molecular model kit and build them. This way, you can rotate them in space and see how the 2-dimensional drawing corresponds to the actual molecule in 3D.
Formation of Epoxides from Halohydrins
Now, moving on to the next common epoxidation technique, we can cause an intramolecular SN2 reaction in vicinal halohydrins. For instance, if I took cyclohexene and treated it with bromine and excess of water, I would get the corresponding halohydrin. Then, if I react this halohydrin with a base of some sort, we’ll deprotonate the alcohol which will cascade into an intramolecular SN2 reaction closing the epoxide ring. If you need a reminder of how this reaction works, I have a dedicated video for the halohydrin formation via the hydroxyhalogenation of alkenes.
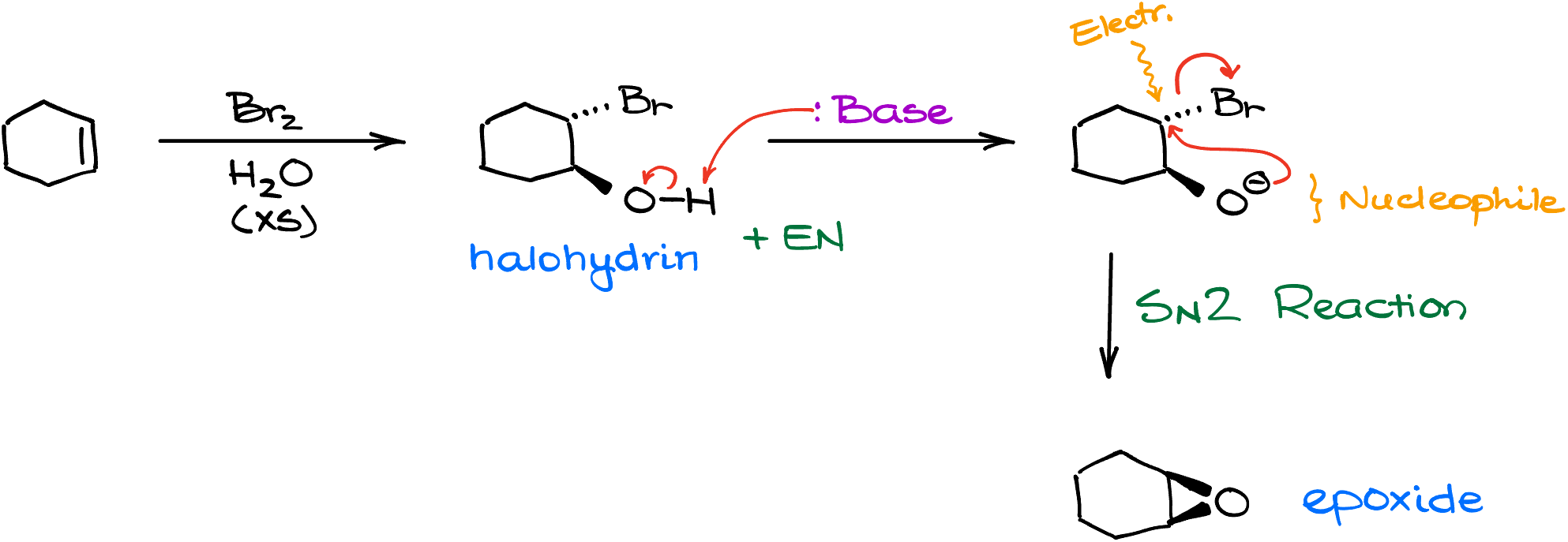
Looking at this reaction, you might have a reasonable doubt about how efficient it is since 3-membered rings are typically quite unstable. True, a three-membered ring is very strained and tends to burst open at the first opportunity. But, due to the fact that the electrophilic and nucleophilic centers in this molecules are so close to each other, the reaction still proceeds comparatively smoothly giving you the epoxide despite how strained it might be.
So when you’re working on your functional group transformations, you can choose whichever method you like. Are those methods completely interchangeable? No, they are not.
Comparison of the Methods
Since both synthetic pathways start from the corresponding alkenes and have the same stereochemical outcome, you might indeed wonder why one would wanna do this two step version over the direct epoxidation with proxy acids. Within the scope of a typical sophomore organic chemistry, we usually don’t go into the limitations of the epoxidation reaction. But nonetheless, they do exist. And the reaction of alkenes with proxy acids sometimes fails. Then, you would wanna use the halohydrin approach.
Also, proxy acids sometime react with other functional groups in your molecule like, for instance aldehydes and ketones in the Baeyer-Villiger oxidation. In this case, it would be a good idea to use the halohydrin over the reaction with proxy acids. However, as I’ve mentioned a moment ago, most instructors and textbooks won’t discuss those details. So, for the vast majority of organic chemistry students, these reactions are indeed interchangeable.
