Ozonolysis of Alkynes
Today, we’re diving into the fascinating world of ozonolysis, but this time, we’re focusing on alkynes. If you’ve already tackled the ozonolysis of alkenes, you’re in luck—alkynes are actually easier to deal with.
The Mechanism We Don’t Need to Know
Let’s start with the reaction mechanism. Actually, let’s not! Surprisingly, you’re not responsible for knowing the mechanism of alkyne ozonolysis. The truth is, even seasoned chemists might stutter if you put them on the spot about it. So, let’s just skip it and jump right into the good stuff—the shortcut to figuring out the products.
A Shortcut to Success
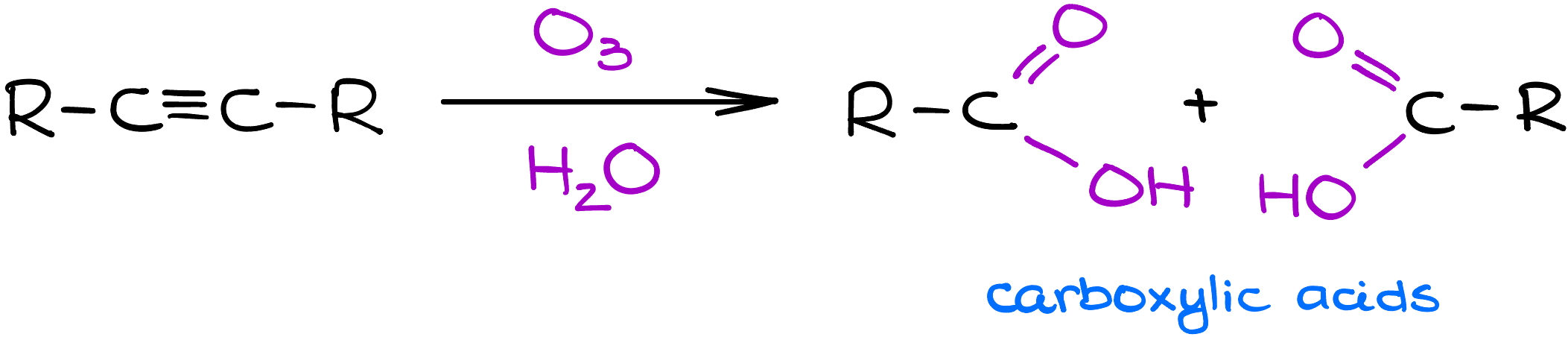
Ozonolysis cleaves the triple bond in alkynes, transforming them into carboxylic acids. But how do you predict these products? It’s easier than you think.
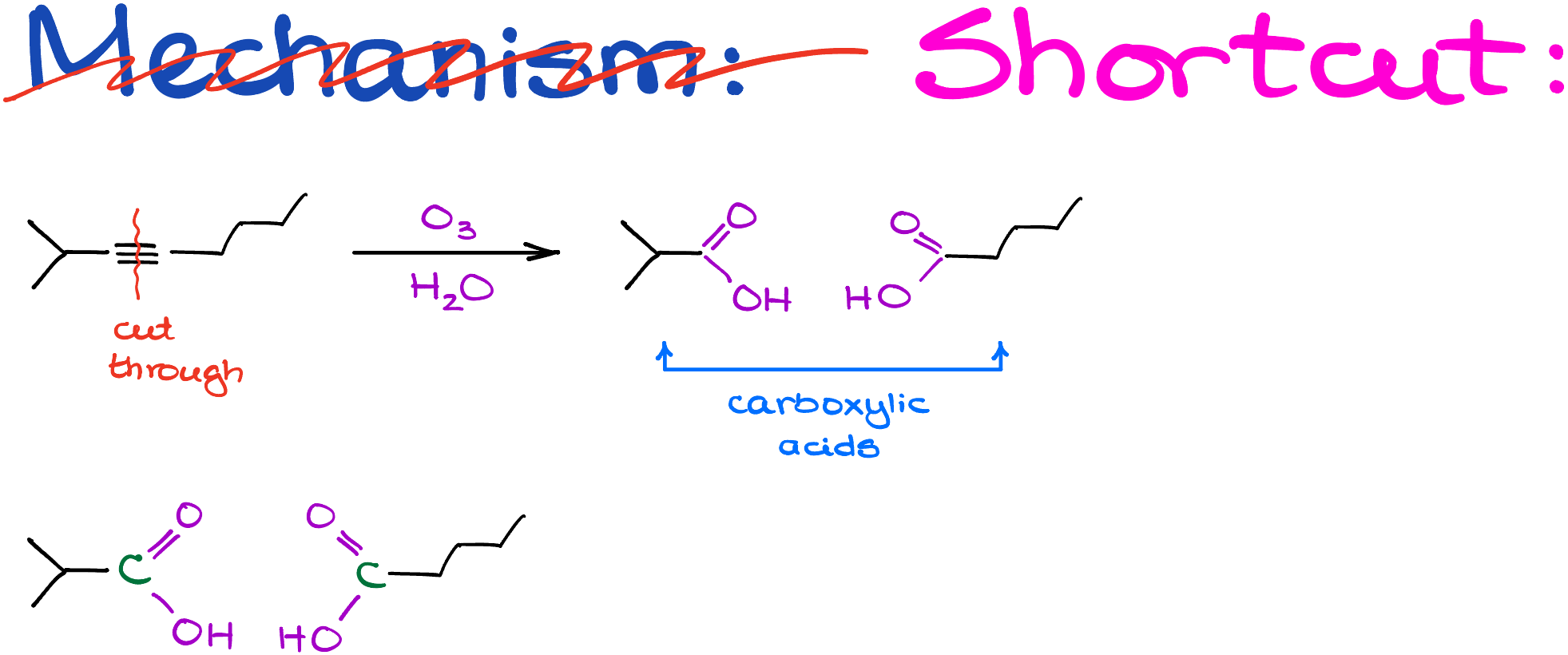
- Start by sketching your alkyne: Take your alkyne and draw it out with a visually exaggerated triple bond.
- Erase the Triple Bond: Sounds destructive, but it’s useful. Also, mark the carbons that were part of the triple bond.
- Add Functional Groups: Simply add a C=O and an -OH to those marked carbons, turning them into carboxylic acid functional groups.
Voila! No extra work-up steps needed. You’ve got your carboxylic acids, neatly derived from your starting alkyne.
Special Cases: Multiple and Cyclic Alkynes
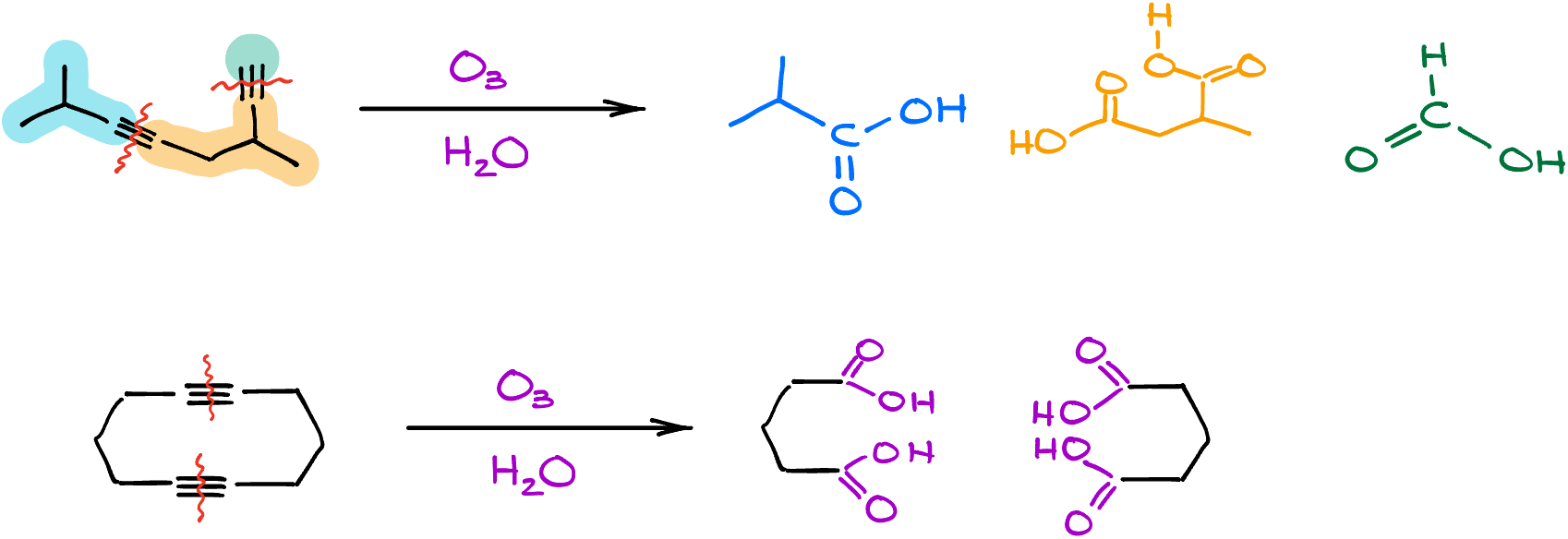
What if you have more than one triple bond? They’ll all get cut, yielding multiple carboxylic acid products. Even cyclic alkynes won’t pose a challenge; they also yield carboxylic acids when cut.
Wrapping It Up
Ozonolysis of alkynes is straightforward—especially if you’ve got a knack for alkenes. Just sketch, snip, and supplement to easily predict your products. No need to memorize mechanisms that even pros find tricky!
