Halogenation of Alkynes
In this tutorial I wanna talk about the halogenation of alkynes.
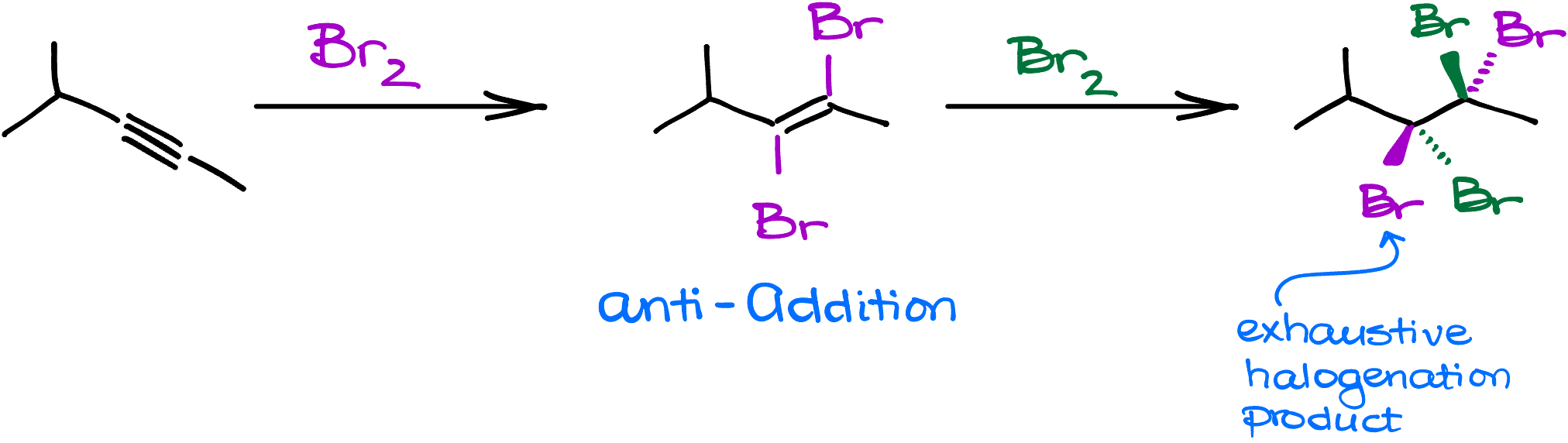
By the end of this tutorial, you’ll know everything you need to know about the mechanism of this reaction, the stereochemistry, and all the tricks your instructor will bring to the test to catch you.
Mechanism of the Halogenation of Alkynes
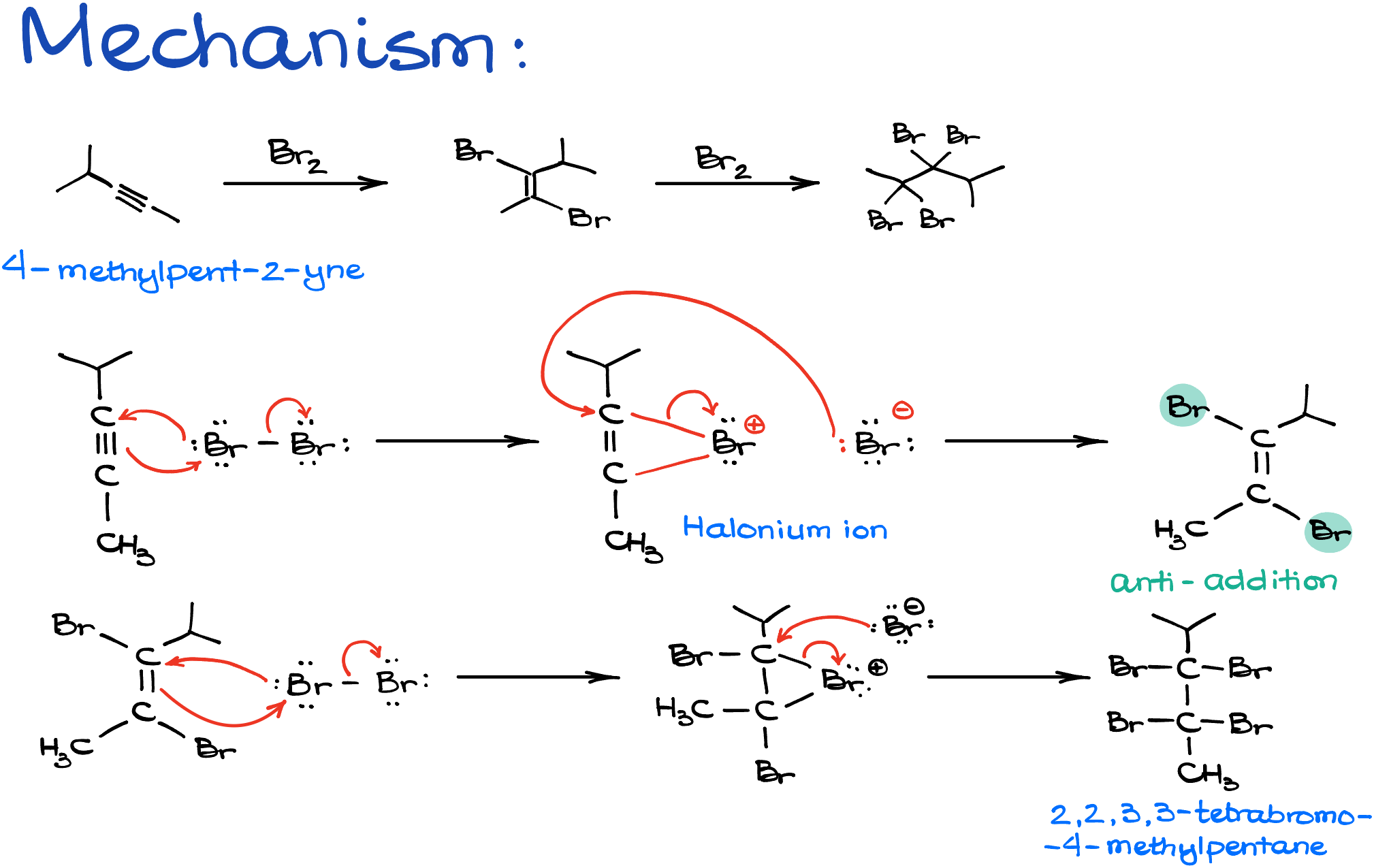
In the halogenation reaction of 4-methylpent-2-yne with bromine, the process begins with the bromine molecule acting as the electrophile, while the alkyne functions as a nucleophile. The nucleophile donates electrons to form a new bond, while the electrophile accepts them. During the reaction, electrons flow from the pi-bond of the alkyne to the bromine, resulting in the formation of a carbon-bromine bond. As bromine can’t accommodate more electrons, its bond breaks, causing one of the bromine atoms to separate as an ion. This leads to the creation of a halonium ion with a positive charge on the bromine atom—a crucial detail to remember for academic purposes. Subsequently, a bromide anion attacks this halonium ion from the opposite side, transforming it into the corresponding alkene. This reaction is specifically an anti addition, resulting in a trans product. This process can be repeated with the newly formed dibromoalkene, which when reacted with another equivalent of bromine, produces another halonium ion. The subsequent bromide attack gives the final product: a tetrahalogenated alkane, specifically, 2,2,3,3-tetrabromo-4-methylpentane.
This reaction, just like everything we teach you about the chemistry of alkynes, is a bit more complicated when it comes to the actual mechanism. The experimental data suggests that this reaction, just like the hydrohalogenation, is most likely a termolecular process and the actual halonium ion is never formed. But, this is the way most textbooks portray this reaction, so this is going to be the way I show it to you as well. At the end of the day, this is what is expected of you on the test, so I see no point to complain about it too much.
Alkene vs Alkyne Reactivity
I also wanna point out that alkenes are generally more reactive than alkynes in this types of reactions. So, we would expect the second step in this process to be somewhat faster than the first step.
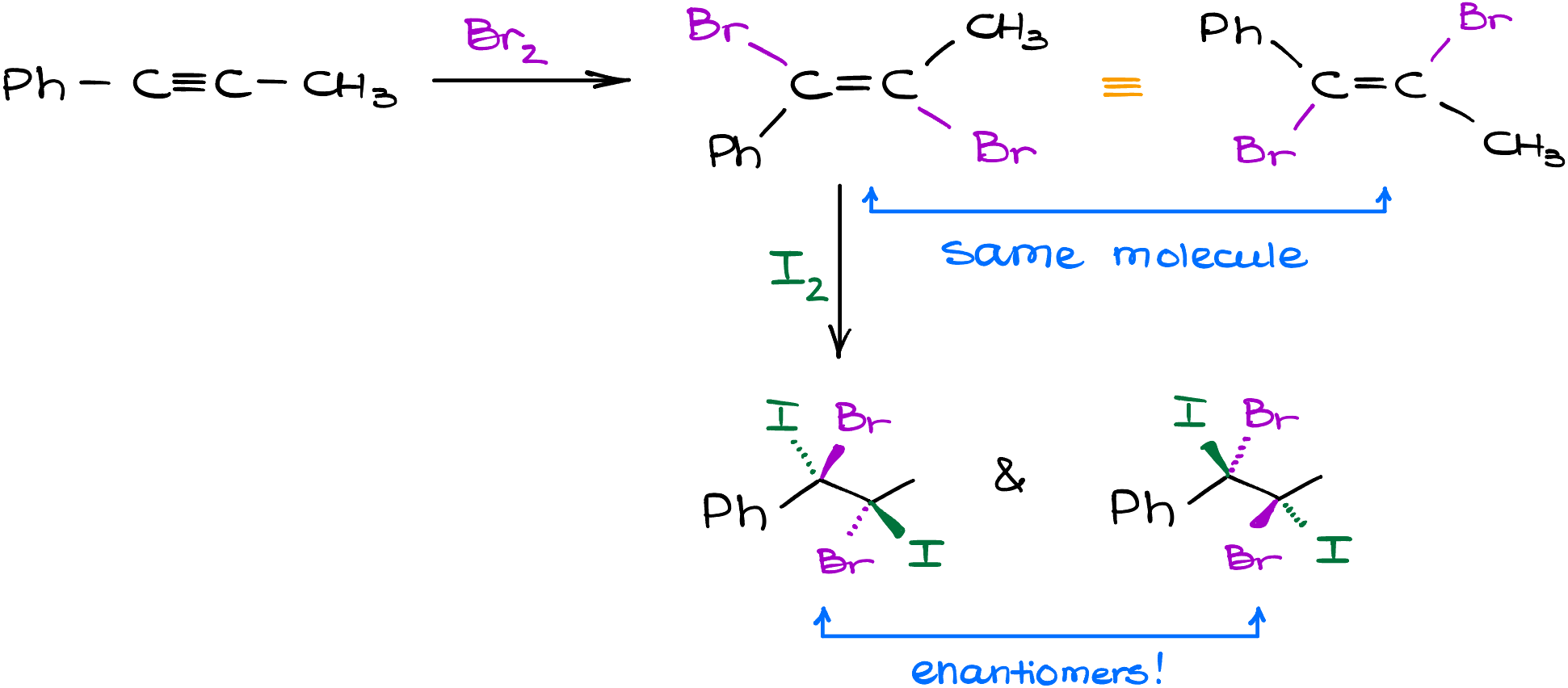
However, while the second step in this reaction is indeed typically faster, it’s still possible to stop it after the first addition and “catch” that di-halogenated alkene. If you’re lucky, you can even then change your halogens for the second step. Instructors love to mix and match those on the test, so be prepared!
Here’s a couple of examples where we can change the halogen in between the steps. When working with these reactions, always make sure you’re paying close attention to the stereochemistry as you can end up with enantiomers, diastereomers, or plain achiral molecules.
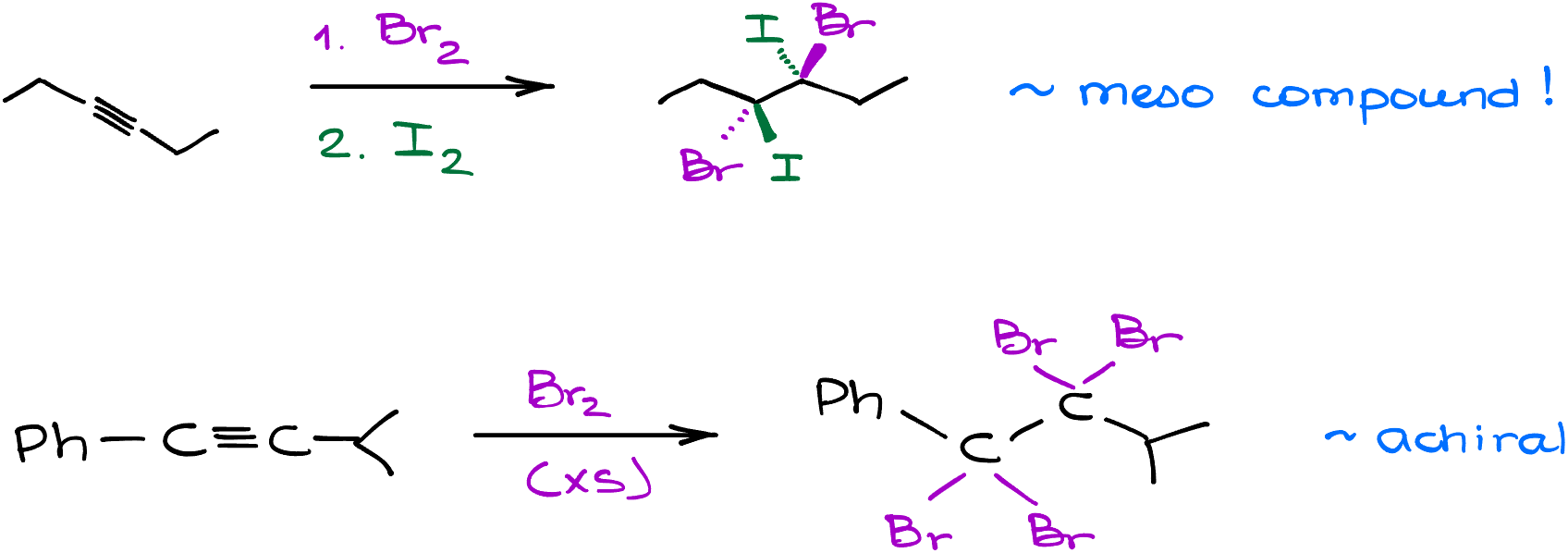
So, while the halogenation of alkynes might seem like an easy and straightforward reaction, it can easily bring a punch to the test if you’re not paying attention.
