Hydration of Alkynes
In this tutorial, we’ll go over the hydration of alkynes mechanism in both acidic conditions and with a mercury catalyst! Hydration is the process where water is added to alkynes.
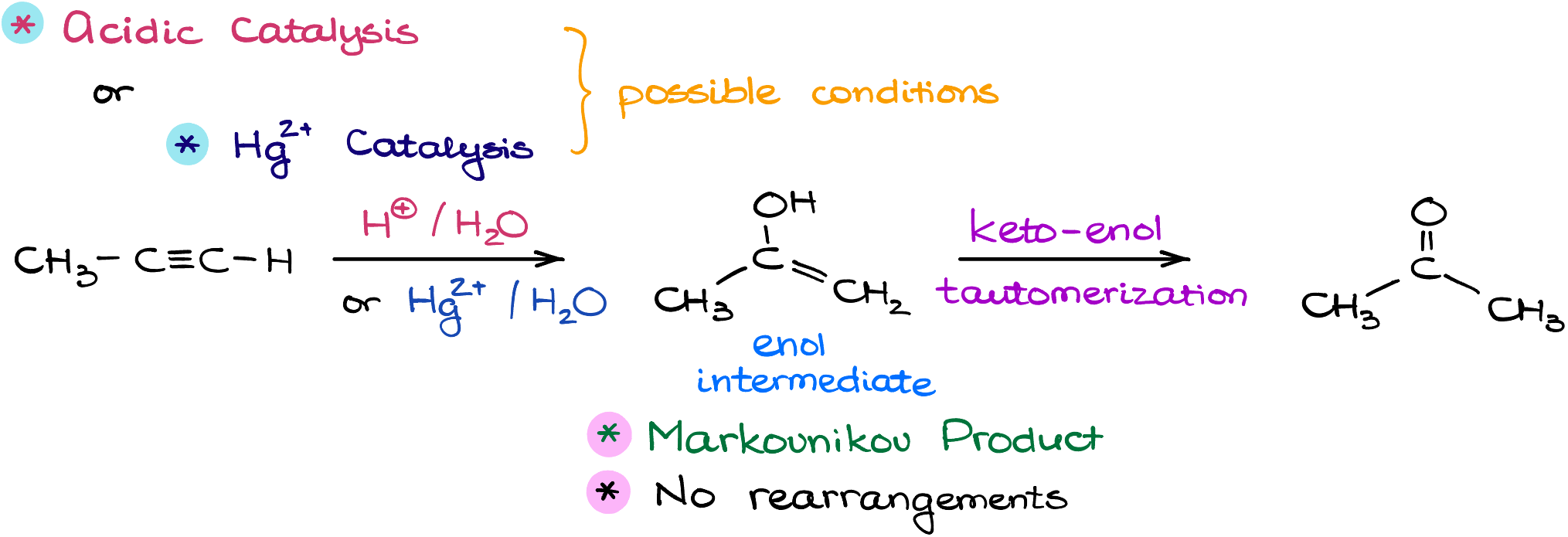
The magic happens typically in two settings:
- Using a Brønsted acid as a catalyst.
- Or, with the gentle touch of catalytic mercury +2 ions, which are a lot kinder to our molecules than pure acid.
Now, whichever path you choose, both will lead you to our intriguing friend: the enol intermediate. But wait, this enol isn’t going to hang around for long! It quickly transforms, undergoing keto-enol tautomerization, and ta-da! We get our final product, the ketone. This entire journey, my friends, is backed by a captivating mechanism that we’ll delve into.
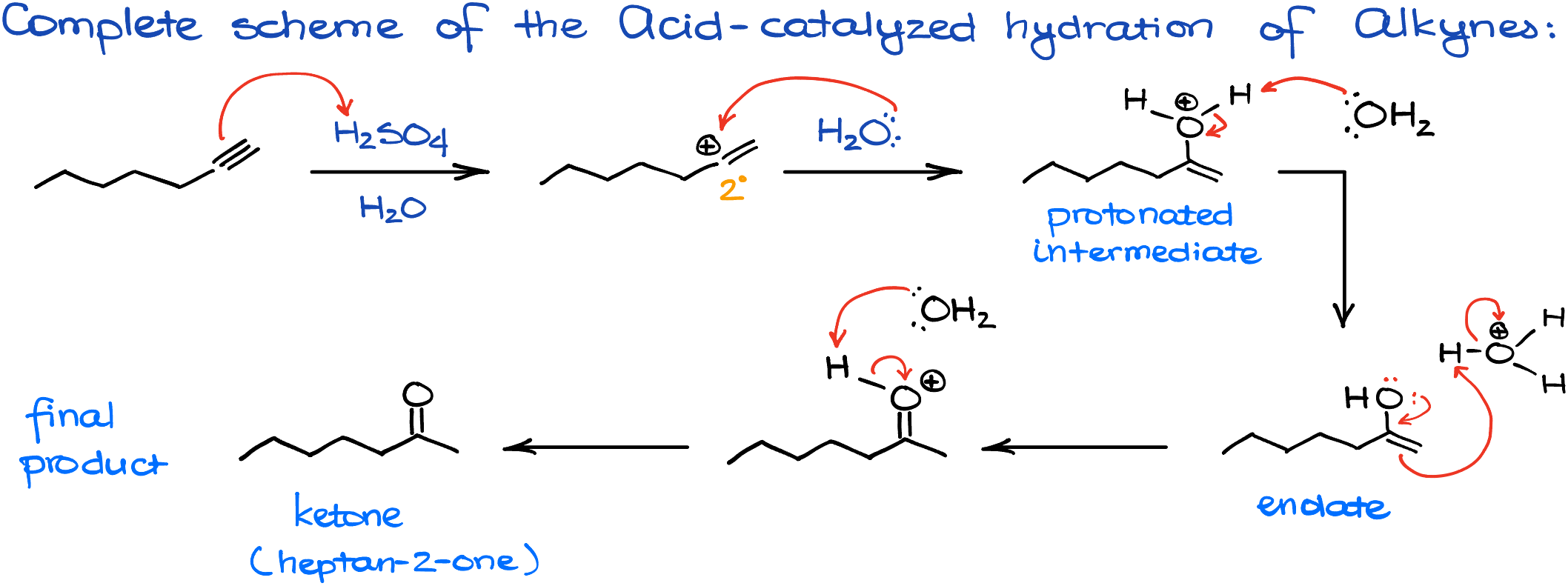
Acid-Catalyzed Hydration of Alkynes
As we delve into the acid-catalyzed mechanism, we’ll be using propyne as our illustrative compound. Given that we’re venturing into the realm of hydration in acidic conditions, sulfuric acid is our chosen acid for the ride. Our journey begins with an electrophilic attack on the alkyne. Now, I know there’s some contention about this mechanism’s details, and while I have my reservations about its portrayal in several textbooks, for the sake of clarity, we’ll adhere to this popular depiction.
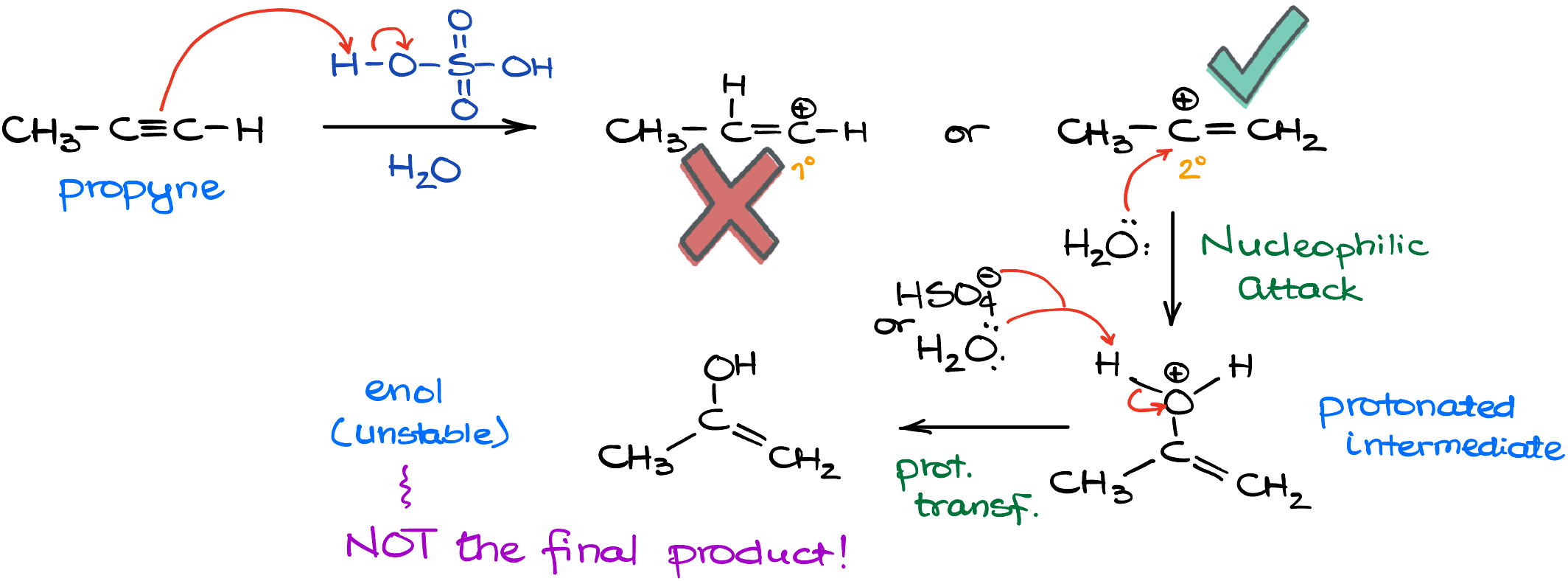
During this electrophilic attack, two outcomes are possible: we can either have the primary carbocation or its more stable sibling, the secondary carbocation. While the primary carbocation might sound appealing to some, we’ll quickly set aside that notion and focus on the more probable secondary carbocation. Following this, the scene is set for a nucleophilic attack, with water playing the role of our valiant nucleophile. This action leads to the formation of a protonated intermediate, which we’ll then deprotonate using another water molecule or the conjugate base of our earlier used sulfuric acid. Voilà, we’ve now reached the enol product. But hang on, this enol is a tad unstable and isn’t our endgame. Remember our earlier chat about the keto-enol tautomerization? Well, this reaction goes through that very transformation, ultimately gifting us with a ketone as the grand finale.
Keto-Enol Tautomerism
So, what’s the buzz about keto-enol tautomerization? To grasp this, let’s first understand the players in the game. A ketone functional group is essentially a carbonyl (that charming C=O double bond) flanked by two alkyl groups. On the other hand, an enol is a slightly more complex beast, characterized by an -OH group that’s cozied up next to a carbon of a double bond.
Now, the term “tautomerism” might sound like something out of a Shakespearean play, but it’s just a schmancy way of saying that these two functional groups, ketone and enol, coexist in a delicate balance. More often than not, this equilibrium is skewed towards the ketone, which is why enols are like those rare guest appearances in TV shows – infrequent and short-lived.
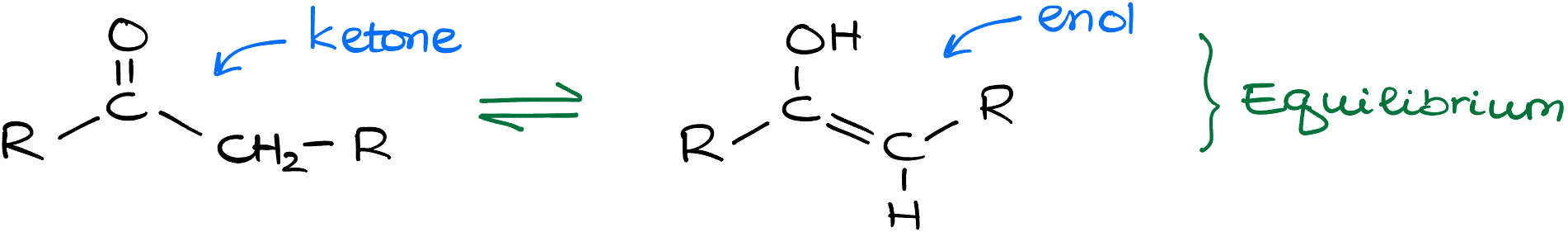
But here’s the catch: if enols are so transient, how does our enol from the previous reactions become the more stable ketone? Great question! Let’s pull back the curtains on this magical transformation.
Keto-Enol Tautomerization Mechanism
Imagine the enol we crafted in our prior escapade. This enol, in the presence of the acid, undergoes protonation, but it’s the double bond that steals the limelight and gets protonated, leaving the oxygen out of this action. This series of events leads us to a protonated intermediate. From here, the story unfolds with either water or our trusty sulfate anion stepping up to deprotonate this intermediate. And voilà, what we’re left with is none other than acetone, in this specific example.
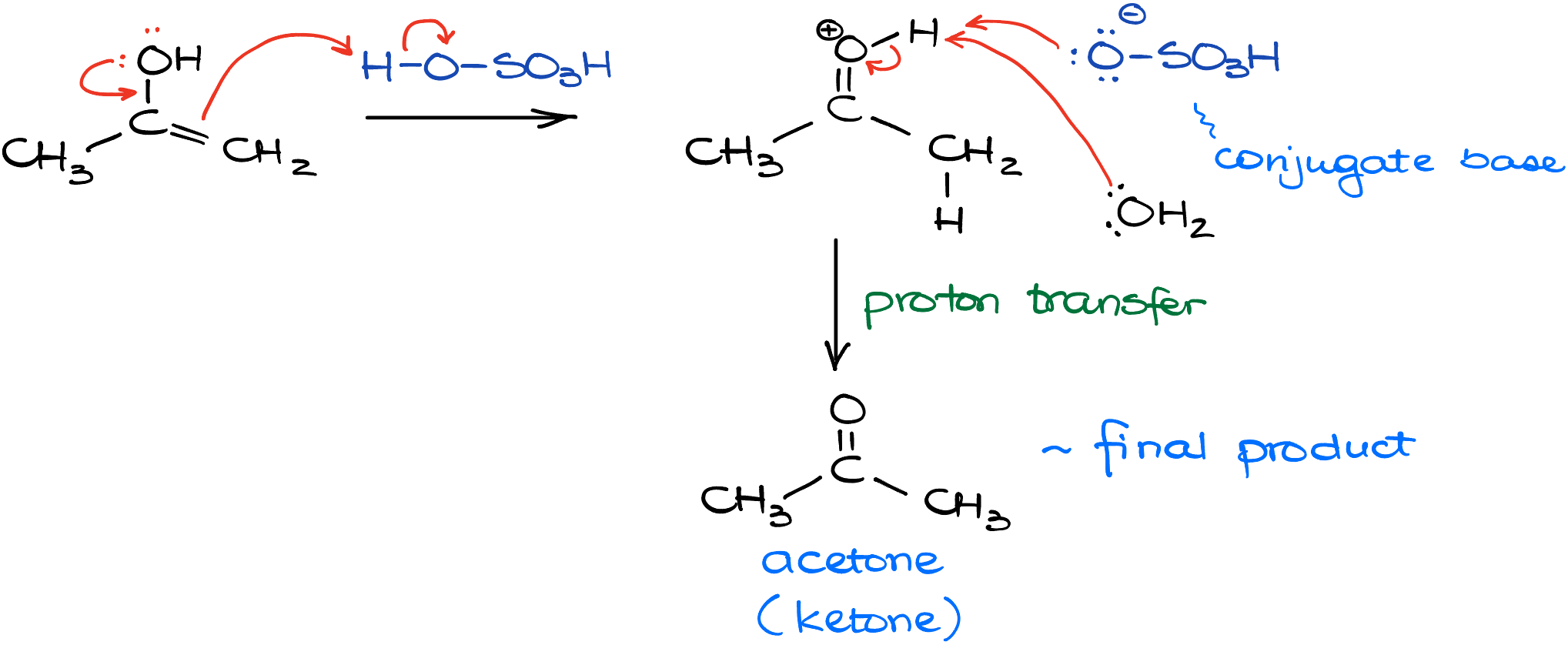
In essence, this transformational dance is all about a shift in the spotlight between the double bond and a hydrogen atom.
A Quick Recap of the Mechanism
So, here’s the entire mechanism from the beginning to the end.
Trick to Predict the Products of the Alkyne Hydration Quickly
You know those cheat codes you find in video games? Well, organic chemistry has its own version! When considering these reactions, you can bypass the intricacies of the enol formation mechanism by keeping in mind that this reaction produces the Markovnikov’s product. Let me break it down.
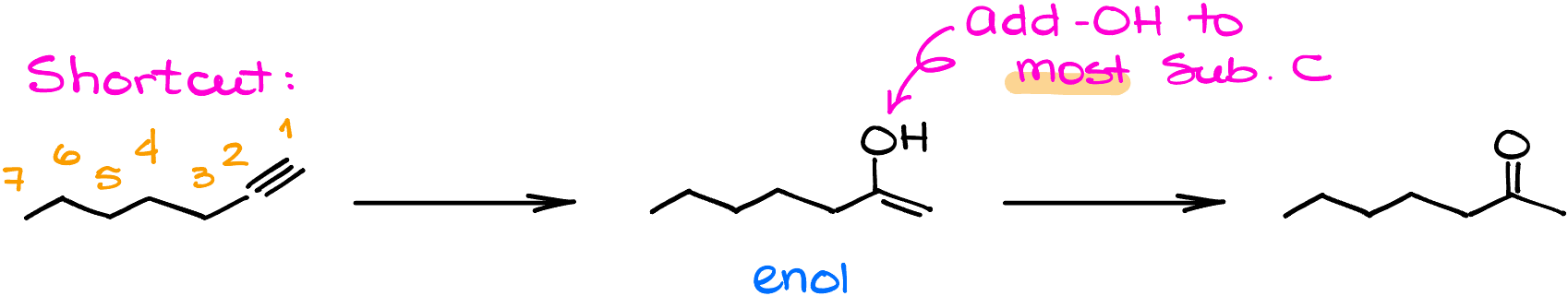
First, take a gander at your starting material and start numbering your carbons, a bit like counting your chess pieces before a game to make sure you don’t lose any atoms in the way.
Next, let’s jazz things up. Introduce a double bond between carbons that used to be your triple bond. It’s a double bond now. Then add the -OH to the more substituted atom. Voilà, you’ve got your enol intermediate!
But, the show’s not over yet. Re-sketch that enol. With a deft stroke, erase the hydrogen attached to the -OH and the double bond. Then, make that carbon and oxygen bond a double bond, making final product—a ketone.
A little word of caution: I’m describing this using bond-line structures, which implies hydrogens on carbons are like silent performers – always present but not always seen. So, when you’re sketching out Lewis structures, ensure you’re not unwittingly getting rid of these vital players.
To encapsulate, the trick is in remembering to add your -OH to the beefiest, or most substituted carbon of the alkyne. Then, in a game of molecular switcheroo, convert it to a carbonyl group while bidding adieu to the C=C bond.
In a nutshell: Add OH, erase C=C, introduce C=O. And there you have it – organic chemistry made simple-dimple!
The Mercury-Catalyzed Hydration of Alkynes Mechanism
The mercury-catalyzed mechanism for alkynes is notably more intricate. We start off with an alkyne as our foundational material. The process commences with an electrophilic attack by mercury. It’s worth noting that depending on your reference material, this step can be illustrated with either the Hg+2 ion alone, mercury acetate (Hg(OAc)2), mercury sulfate (HgSO4), or several other variations. For our discussion, let’s use the mercury ion as a representation.
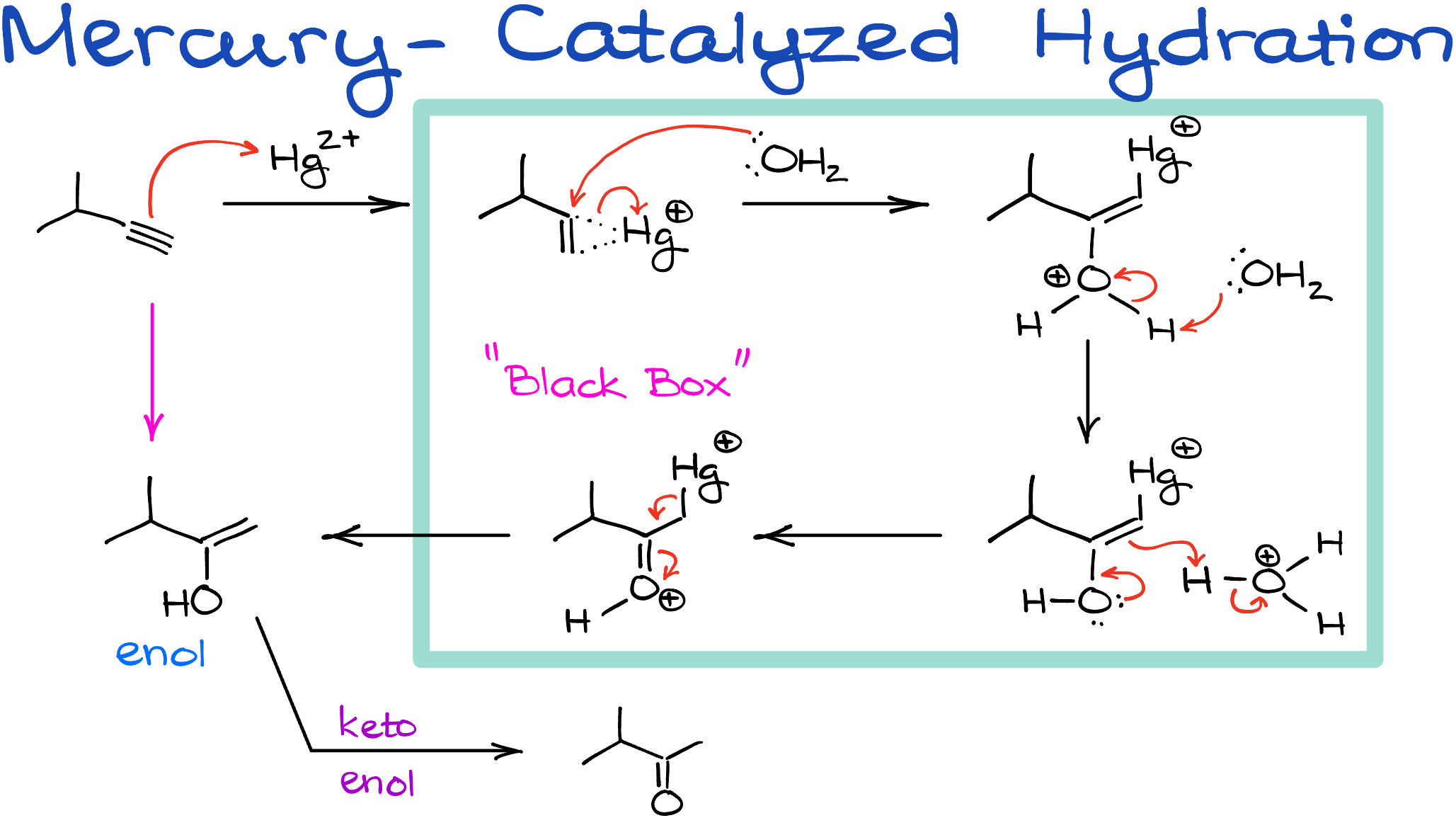
This electrophilic attack results in the formation of a mercuric ion, often referred to as a mercurinium ion. This step closely mirrors the oxymercuration process observed in alkenes. It’s essential to be familiar with this reaction, especially if you’ve covered alkenes in your course.
Subsequently, this ion undergoes a transformation from its more substituted side, leading to the creation of a protonated intermediate. This intermediate, through a series of mechanistic maneuvers, eventually morphs into an enol. Generally, this section of the mechanism is not frequently expected of students, but it’s always prudent to check with your instructor or course requirements.
However, it’s important to note that when we venture into the realm of keto-enol tautomerization, it’s a fundamental topic. We’ve already covered the underpinnings of this mechanism. So, as a hands-on exercise, try sketching the mechanism for the displayed transformation and check if your resulting product aligns with ours. This should involve a two-step process: starting with the protonation of the double bond and culminating in the deprotonation of the intermediate to produce the ketone.
Concluding Thoughts
In this tutorial, we explored the hydration of alkynes. This reaction can be catalyzed using either a Brønsted acid or mercury ions. Both pathways lead to the formation of an enol intermediate. This enol quickly undergoes keto-enol tautomerization to produce a ketone. When examining the acid-catalyzed mechanism, we utilized propyne with sulfuric acid. The mechanism involved electrophilic attack, formation of carbocations, nucleophilic attack by water, and subsequent tautomerization to a ketone. The keto-enol tautomerization mechanism was elucidated, detailing how enols convert to ketones. Lastly, the mercury-catalyzed mechanism was discussed, beginning with an electrophilic attack by mercury, leading to a mercurinium ion and eventually resulting in an enol. This enol, similar to the acid-catalyzed route, undergoes tautomerization to give the final ketone product. In summary, the hydration of alkynes, whether acid-catalyzed or mercury-catalyzed, consistently produces ketones via enol intermediates.
