Cyclopropanation of Alkenes and the Simmons-Smith Reaction
In this tutorial, we’re exploring two fascinating reactions: Cyclopropanation and the Simmons-Smith Reaction. Though distinct, both reactions share a common objective—they transform alkenes into corresponding cyclopropanes. While this may seem like a specialized topic, it’s a frequent subject in exams and an essential concept for any organic chemistry student.
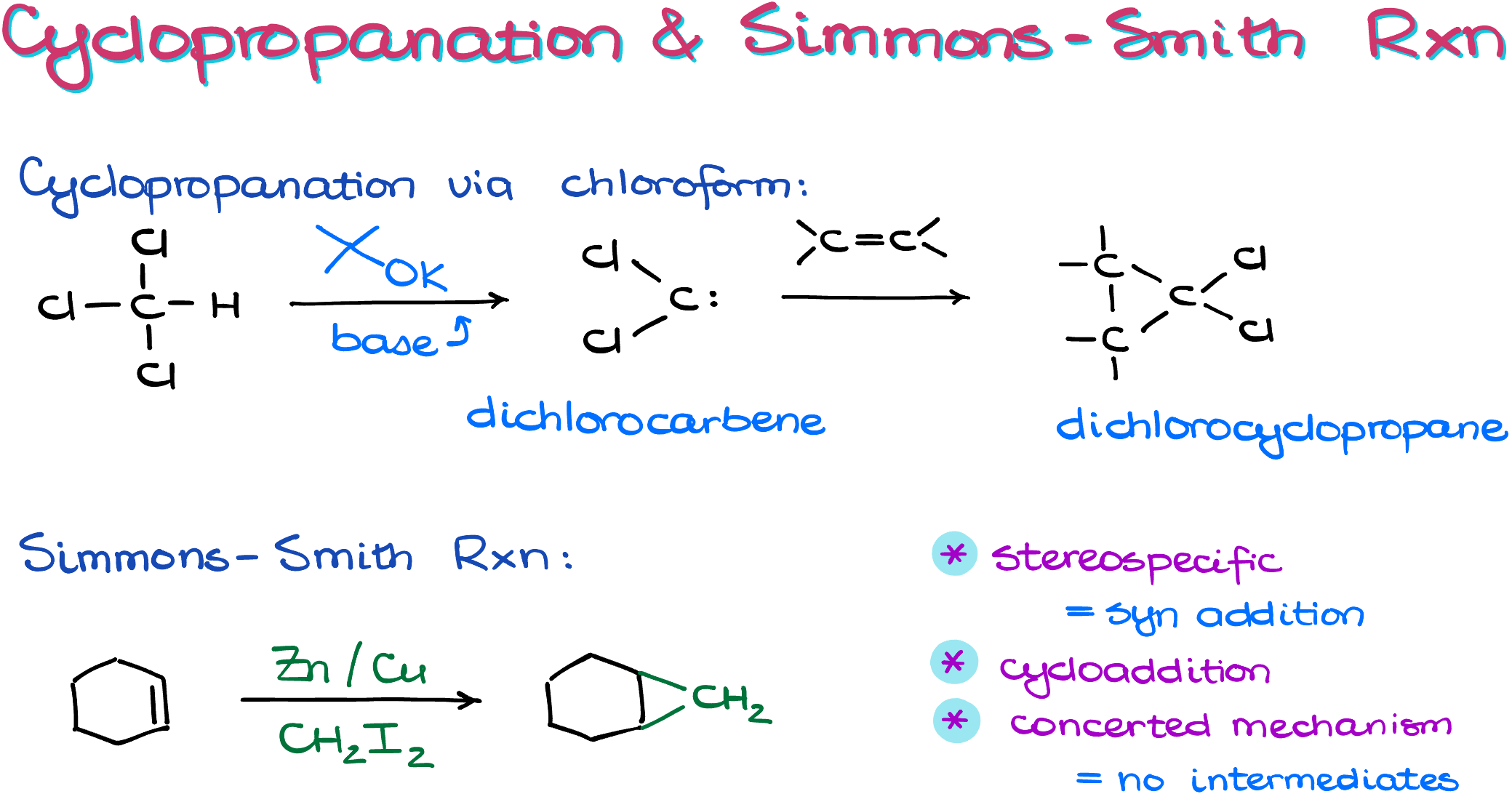
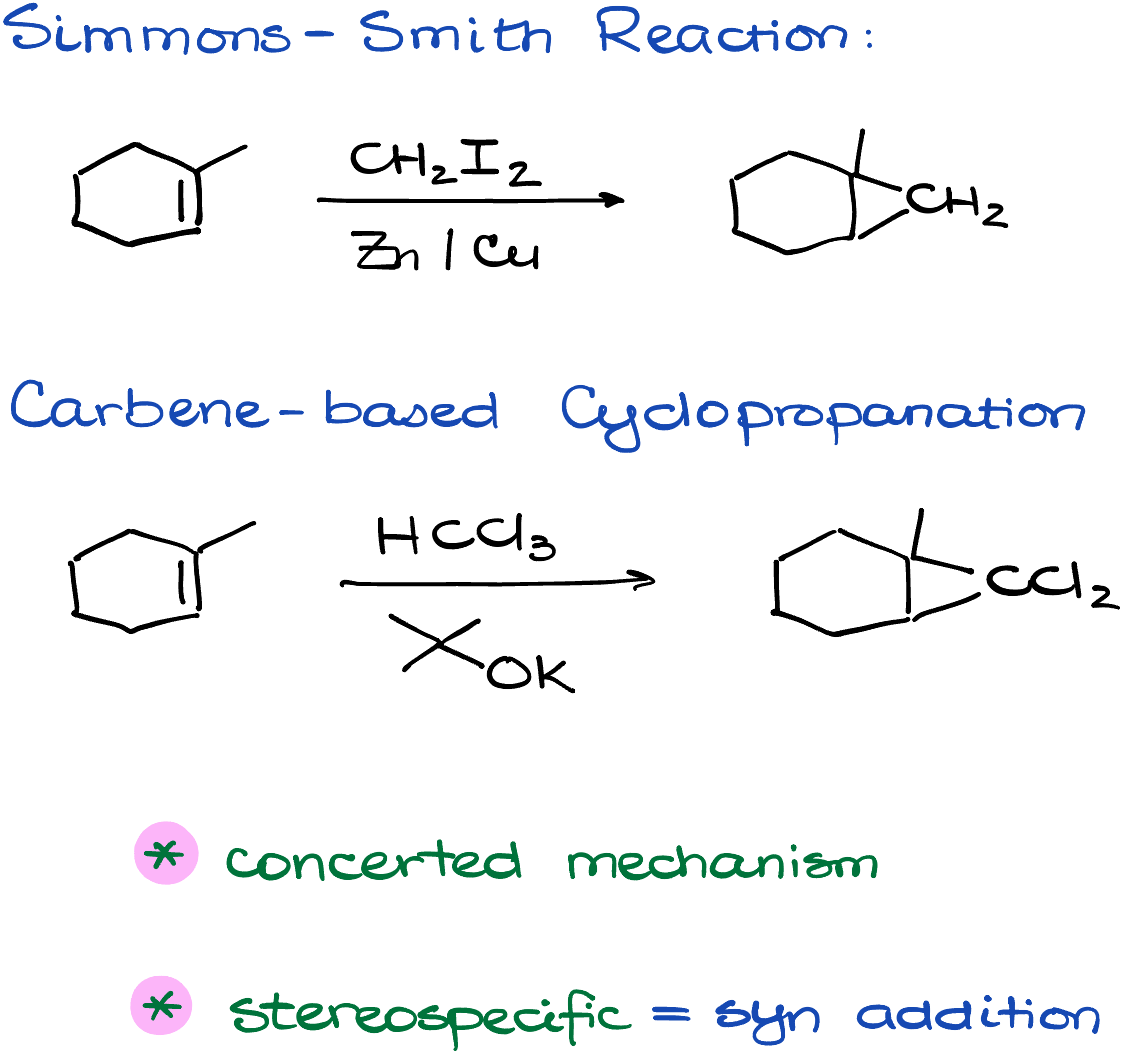
The Simmons-Smith Reaction
The Simmons-Smith reaction utilizes diiodomethane and a zinc-copper alloy to create a carbenoid species. Here’s what the process looks like:
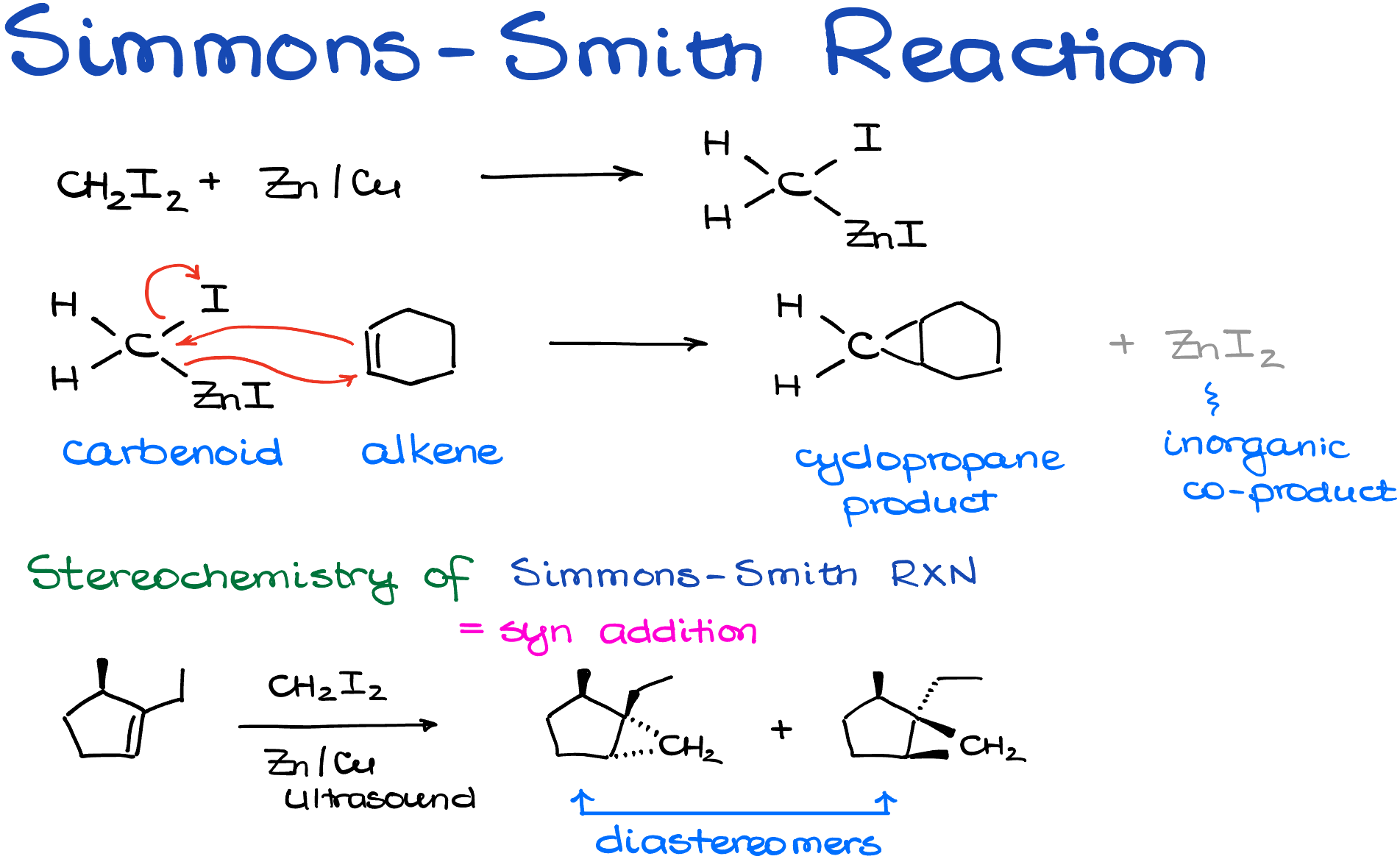
While the exact details of the formation aren’t critical to understand, it’s useful to note that a zinc atom will insert itself between the carbon and iodine atoms, forming a new, reactive species. The quality of the zinc surface can influence the reaction, and ultrasound is often employed to facilitate the process.
Reaction with Alkene
Once we have our carbenoid species, it reacts with an alkene through a concerted mechanism. This creates two new bonds between the CH2 of the carbenoid and each atom in the alkene. The end result? A cyclopropane product.
Byproducts
As for byproducts, zinc iodide is formed but is generally considered irrelevant for our purposes. Therefore, it will be omitted in future examples.
Stereochemistry
From a stereochemical standpoint, the Simmons-Smith reaction is stereospecific and can be categorized as a syn addition. This makes intuitive sense; you can only add a CH2 group to your molecule to form a three-membered ring from one side.
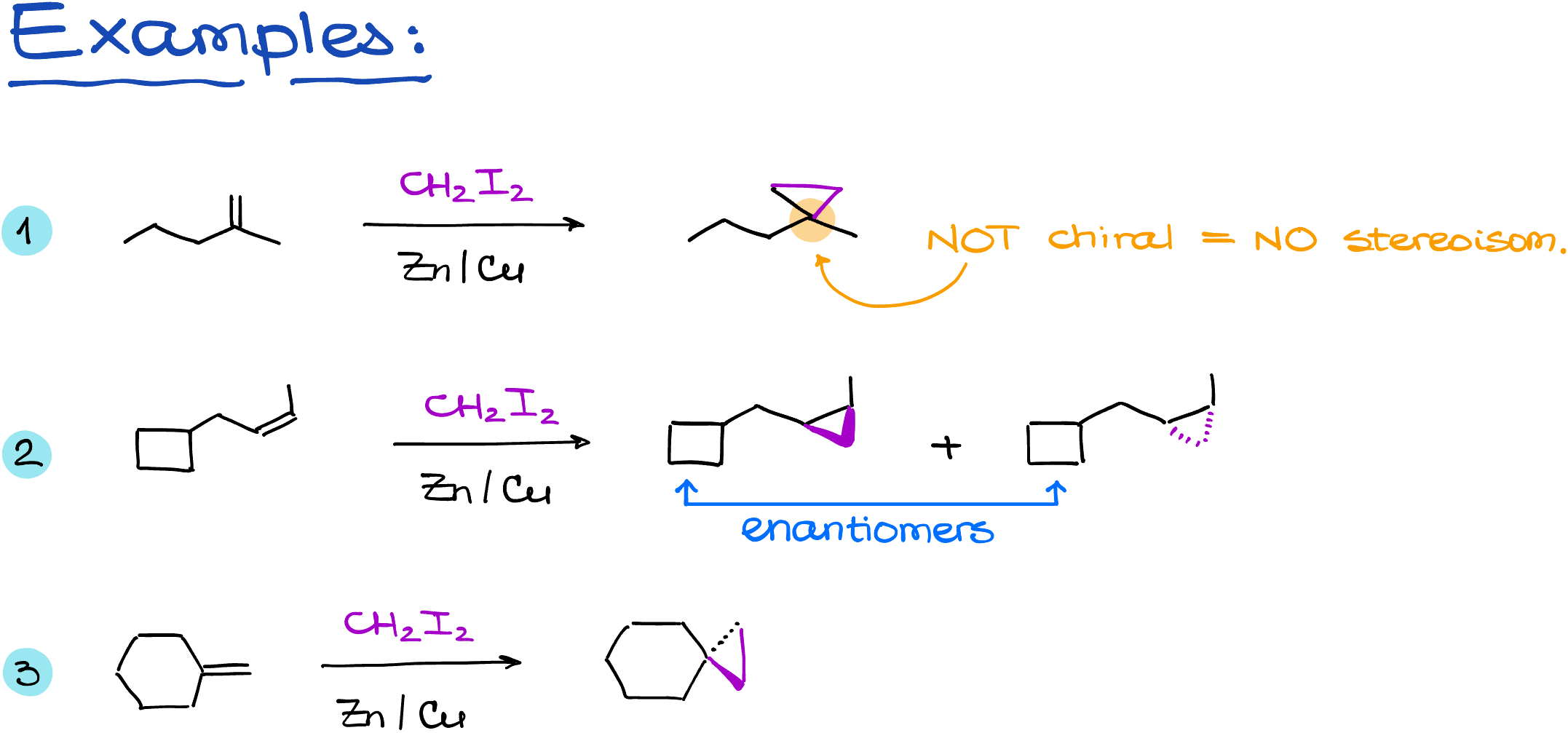
Here are some more examples of the Simmons-Smith reaction:
Cyclopropanation via Carbenes
The second type of cyclopropanation reaction we’ll explore doesn’t come with a specific name, but it starts with the formation of a carbene. There are various methods to generate carbenes, and interestingly, my journey into the realm of chemistry began with the study of carbenes, so this to pic is near and dear to my heart. For the purposes of an introductory organic chemistry course, we commonly create carbenes from chloroform, typically using sodium or potassium tert-butoxide as a base to promote the reaction.
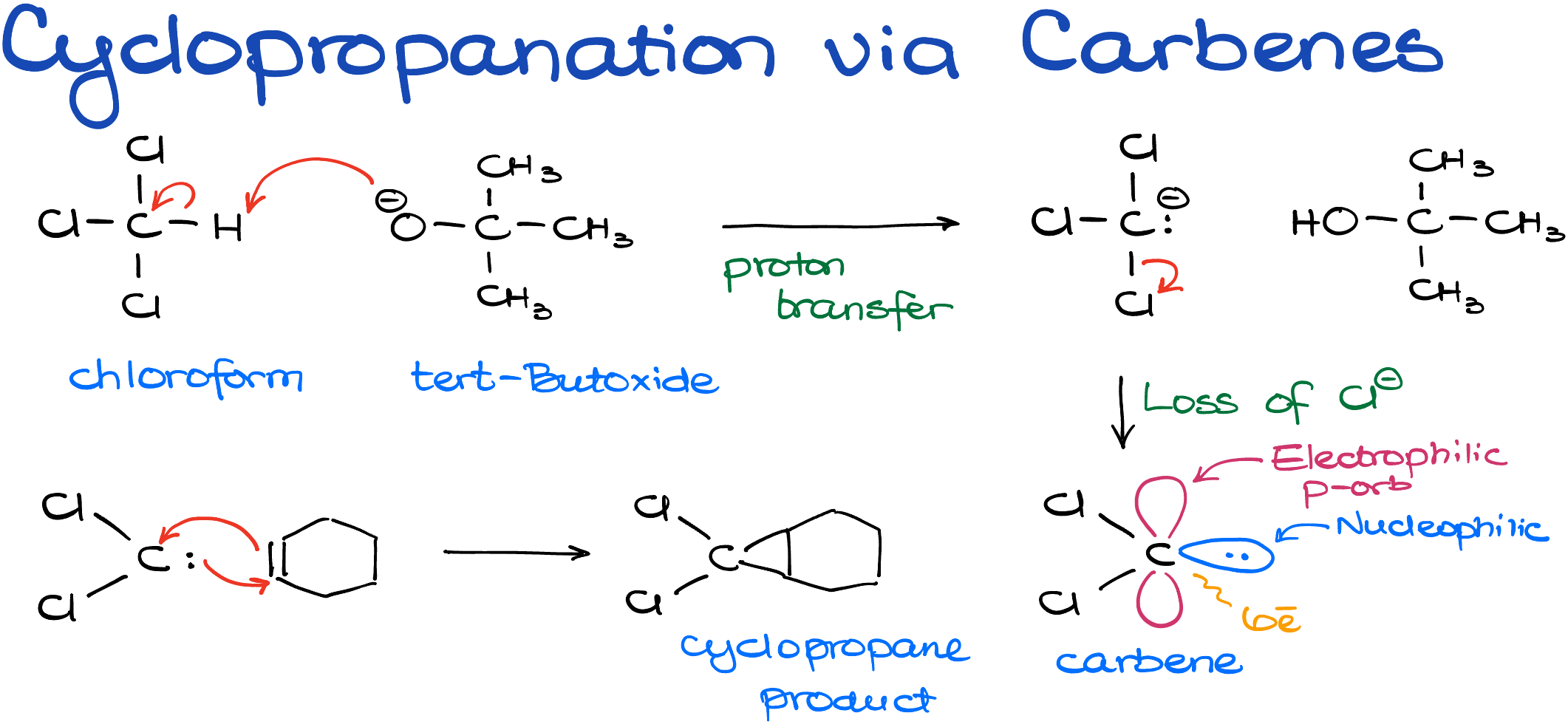
Formation of Carbene
The reaction initiates with a simple acid-base proton transfer. Here, tert-butoxide deprotonates chloroform, creating an anionic species. This chloroform anion then undergoes a chloride loss, resulting in the formation of a carbene.
Understanding Carbene
Carbenes are quite unique: they are neutral, six-electron species of carbon that possess dual characteristics. They act as nucleophiles due to an electron pair they carry, while simultaneously serving as electrophiles owing to an empty p-orbital—truly versatile molecules, chemically speaking.
Interaction with Alkene
In the presence of an alkene, the carbene displays its dual nature. It can both accept electrons due to its electrophilic nature and donate electrons because it’s a nucleophile. This leads to the formation of a new cyclopropane ring.
Notably, the resulting cyclopropane contains chlorine atoms, which may prove useful for subsequent chemical reactions.
Here are a few example of the “regular” cyclopropanation reaction:

Summary and Takeaways
In summary, we’ve delved into two types of reactions that transform alkenes into cyclopropanes: the Simmons-Smith reaction and a general cyclopropanation via carbenes.
Simmons-Smith Reaction
The Simmons-Smith reaction employs zinc and diiodomethane to convert alkenes into cyclopropane rings through a zinc-containing carbenoid species.
General Cyclopropanation
On the other hand, the more general cyclopropanation method uses a carbene intermediate to form a cyclopropane ring, which also features two geminal chlorine atoms.
Similarities
Both reactions operate through a concerted mechanism, meaning they add to the alkene in a single, synchronous step. Additionally, both are stereospecific, resulting in syn addition products.
By gaining a solid understanding of these two types of cyclopropanation reactions, you’ll be better prepared to tackle related questions on exams and engage in practical applications.
