Enantiomers and Diastereomers
Enantiomers and diastereomers are the only two stereochemical relationships that you can have between any two molecules. The stereoisomers are any two molecules that fulfill the following two requirements:
- Both molecules must have the same molecular formula, and
- Both molecules must have the same atom connectivity.
So, what’s the difference then? The molecules are stereoisomers if they have a different 3D shape and they are not superimposable in space.
What does non-superimposable mean?
Non-superimposable molecules cannot be made to look exactly the same no matter how many rotation operations you do with them. The “rotations” also include the free rotation around the single bonds.
Think about your hands. If you align them together so that all fingers line up, the palms are going to be looking in the opposite directions. If you make your palms look in the same direction, your thumbs will be looking in different direction, etc. No matter how much you rotate your hands, you’ll never be able to make them look exactly the same—your hands are non-superimposable mirror images. Same principle applies to molecules.
Then, why do we need two relationships? Why do we need to have enantiomers and diastereomers and can’t just do with one—stereoisomers? There’s actually a different type of relationships that two molecules can have. Let’s dig a bit deeper.
What are the enantiomers?
Enantiomers are two molecules that are non-superimposable mirror images. Just like your hands, molecules may have a mirror image that won’t be superimposable with the original molecule. Look at these two molecules:
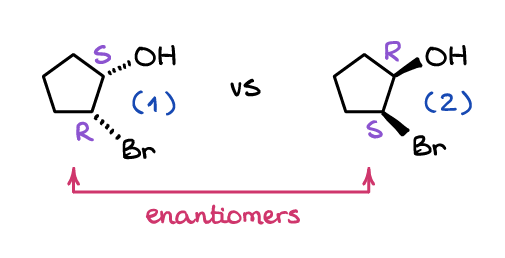
No matter how much you rotate one in space, you’ll never be able to make it look the same as the other one. While molecule (1) and molecule (2) have both groups (OH and Br) cis to each other, they look in different directions from the plane of the cycle.
Note, there are many ways of how you can make a mirror image for a molecule. Here are the three possible examples:
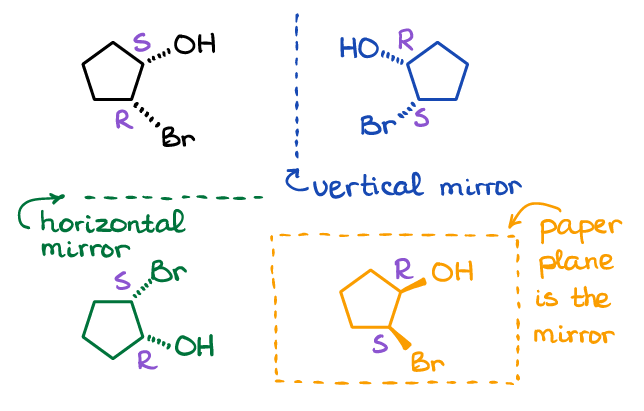
The mirror plane can be vertical, horizontal, or even the paper where you draw the molecule can be the mirror plane itself. I suggest you make a couple of the molecules from the picture above using your molecular model kit (pick one reflection), and make sure they are not superimposable by physically rotating them in space.
Another important distinction of the enantiomers is that all stereocenters are mirrored between the molecules as well. Meaning, all S stereocenters are R’s in the other molecule and all R’s in one molecule are S’s in the other. For instance, the molecule (1) from above is (1S, 2R)-2-bromocyclopentanol, while its enantiomer, molecule (2), is (1R, 2S)-2-bromocyclopentanol.
What are the diastereomers?
When it comes to diastereomers, those are, well—not enantiomers. 😹 I mean, seriously, the “common” definition of a diastereomers is the stereoisomers that are not enantiomers. The official definition though is the diastereomers are non-superimposable molecules that are not mirror images of each other. For instance, let’s look at the following two molecules:
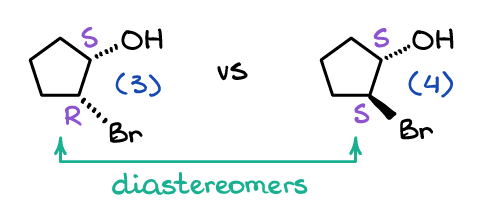
Molecules (3) and (4) are obviously not mirror images, so they cannot be enantiomers. They are also not superimposable in space no matter how much you rotate those in space, so they are not the same molecule either. Thus, by definition, they are diastereomers as they are non-superimposable not mirror images of each other.
Notice, that unlike enantiomers, diastereomers only have some of the stereocenters change from one molecule to the other. For instance, molecule (3) is (1S, 2R)-2-bromocyclopentanol, while its diastereomer is (1S, 2S)-2-bromocyclopentanol.
Do you have to have chiral carbons to have enantiomers and diastereomers?
No, you do not! Note how the definition of enantiomers says that the molecules are non-superimposable mirror images, while diastereomers are non-superimposable non-mirror images? The definitions say nothing about the chiral centers or atoms. Thus, any pair of molecules that fits the definition, works! For instance, allenes are cumulated alkenes that are not planar:
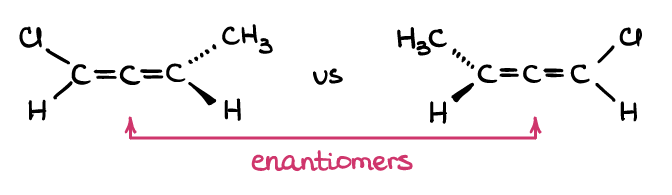
If you build a pair of allenes with your molecular model kit (yes, get your molecular model kit and actually build those!), you’ll see, that those are not superimposable in space. But these two molecules have no chiral carbons… and yet, they fit the definition of enantiomers, therefore they are a pair of enantiomers! So, as you can see, the chiral atoms by themselves is not the important thing here, rather it’s the 3D structure of the molecule itself.
You can also have diastereomers in molecules that don’t have any chiral atoms. Look at the following examples:
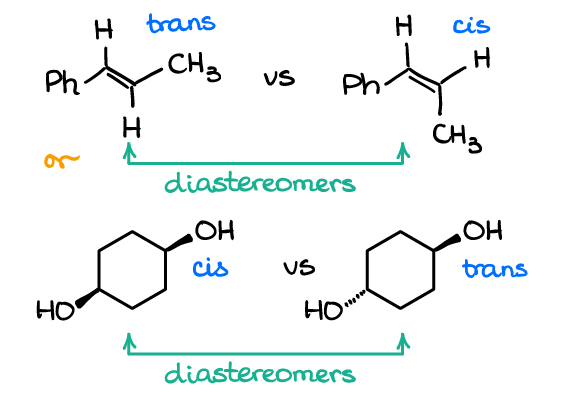
The top pair is an example of the cis/trans (or E/Z if you want to use the strict UIPAC names) isomers in alkenes. The second pair also represents as cis/trans pair of isomers. Neither of molecules, however, have chiral atoms. And since each pair represents a couple of non-superimposable molecules that are not mirror images, they are diastereomers.
These are typical examples on tests and many instructors love throwing those questions at you. Many students tend to have a sort of a tunnel vision when it comes to stereochemical relationships focusing only on molecules with chiral atoms. This is a faulty heuristic! So, make sure you always analyze the entire molecule and use the definition of the relationship, rather than only focusing on the chiral atoms.
