SN1 Reactions
Let’s delve into this tutorial about SN1 reactions.
The Mechanism of an SN1 Reaction and the Role of Solvents
An SN1 reaction is a first-order reaction, meaning that the rate of the reaction depends solely on the concentration of the substrate. We can represent this rate as rate=k[Rx], where Rx typically signifies our substrate, the alkyl halide.
Let’s consider the following example:
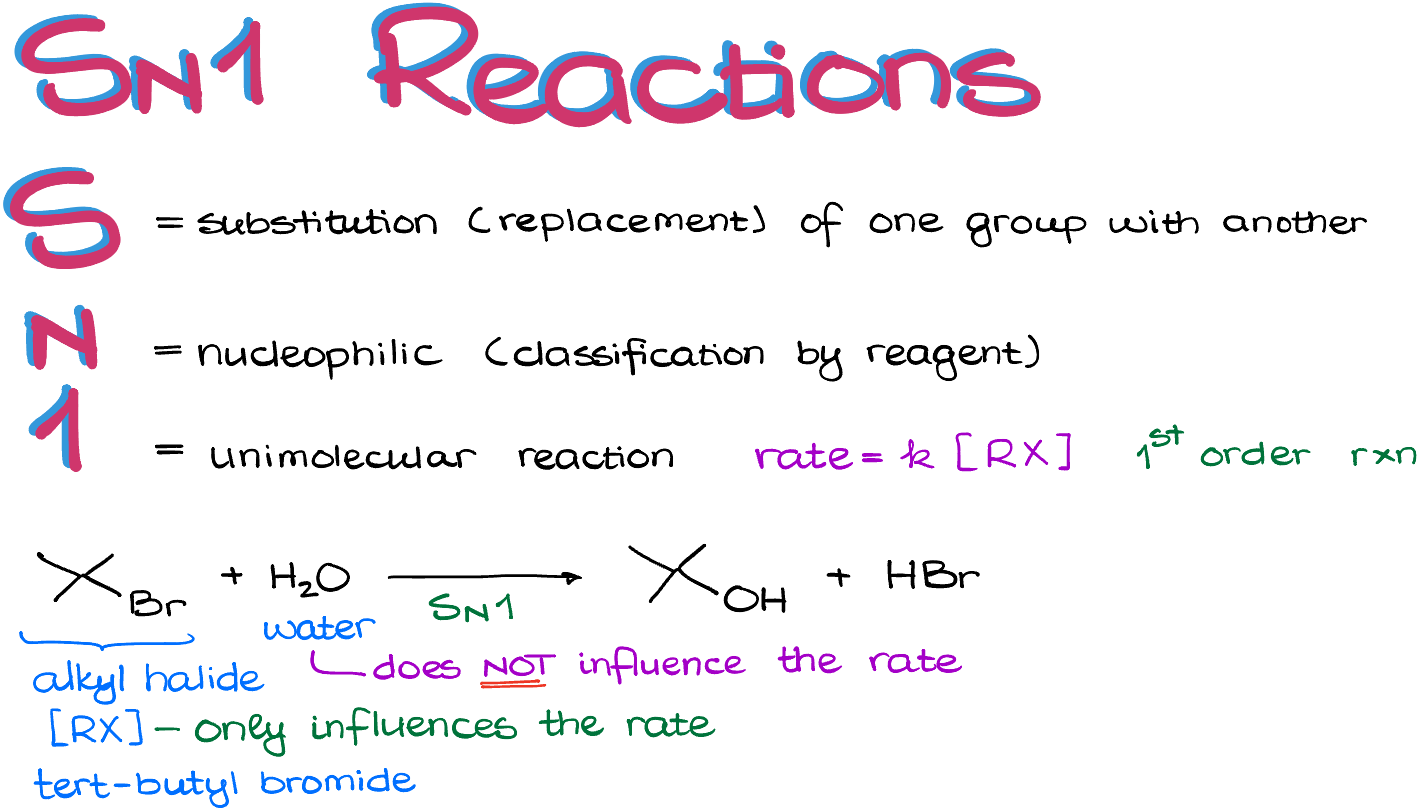
Here, we have the alkyl halide and H2O as our two reagents. However, the reaction rate only depends on the concentration of the bromide, not the water. Now, let’s examine the mechanism of this reaction. The first step involves the leaving group (LG) dissociating, which results in the formation of a carbocation intermediate:
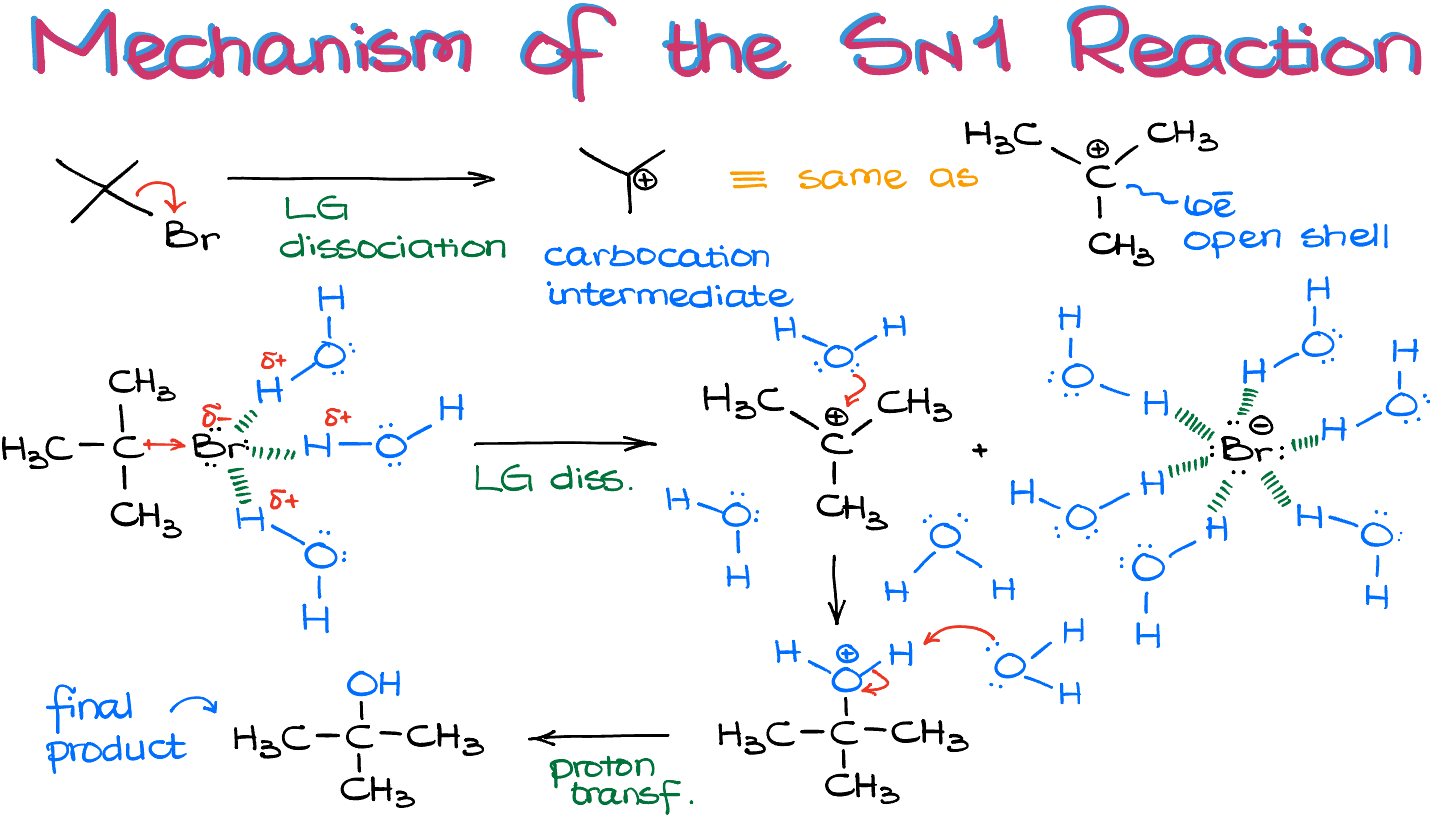
It’s common knowledge that bond-breaking is an endothermic process. So, why does this process occur and why doesn’t the bromine atom (Br) simply reattach, bringing the reaction back to its starting point? This is where the solvent becomes crucial!
SN1 reactions favor polar protic solvents like H2O, ROH (alcohols), or acids. The LG, in this case Br, carries a negative charge, which attracts the partially positive hydrogen atoms from the solvent. This creates a solvation layer around the Br. As bonds naturally vibrate, the pull from the hydrogen atoms strengthens the vibration, eventually detaching the Br from the molecule. As the Br leaves, the polar protic solvent surrounds it, forming a solvation shell that stabilizes the Br and prevents it from reattaching to the carbocation.
With the carbocation now surrounded by water molecules, the next step is the nucleophilic attack by H2O onto our carbocation, resulting in a protonated species. We generally want to remove the positive charge on the oxygen atom by taking away the extra proton with either Br or another H2O molecule, with the latter being statistically more likely.
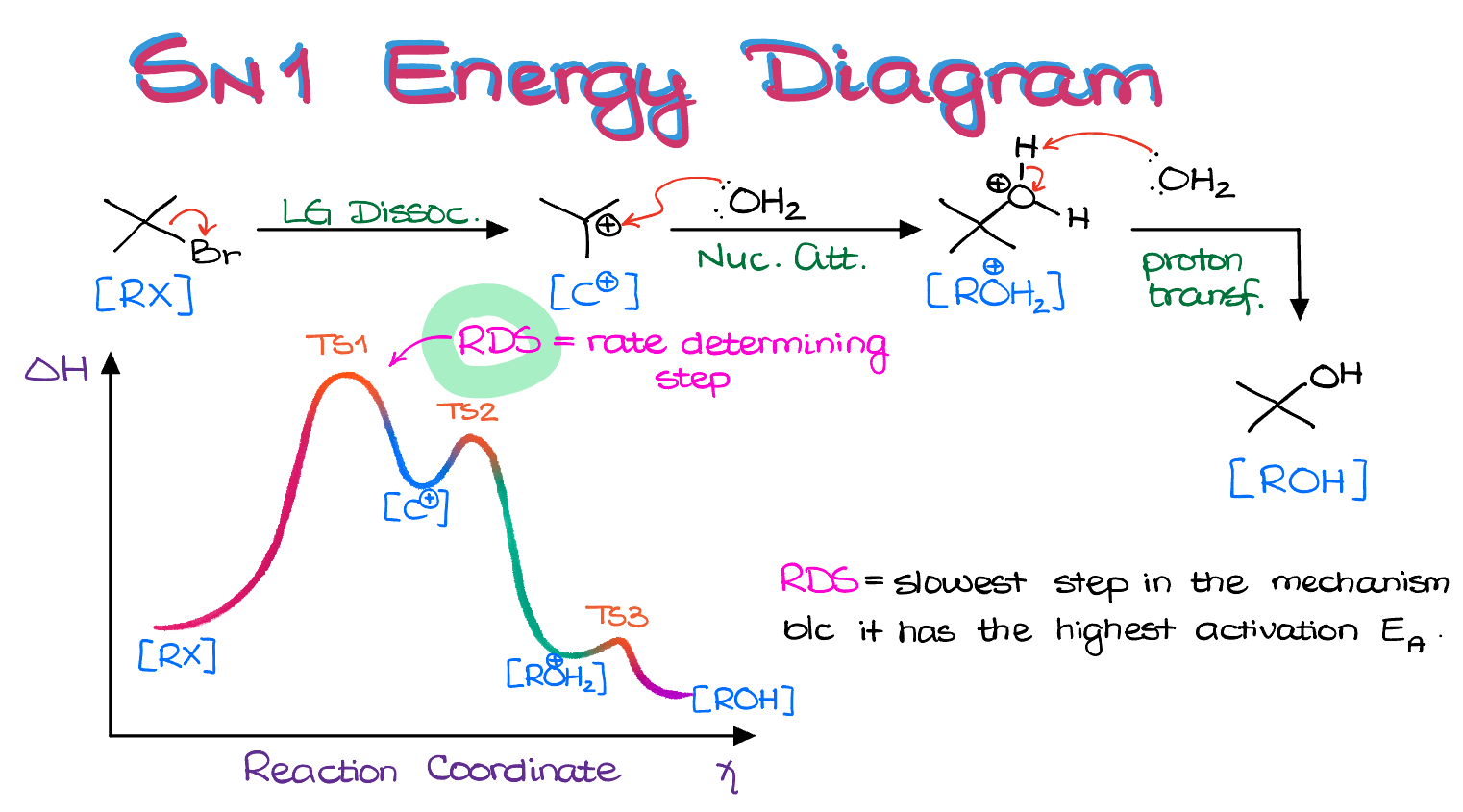
Energy Diagram of an SN1 Reaction
When viewing this reaction from the energy diagram perspective, we see three steps. The rate-determining step (RDS) is the initial step, leading to the formation of the carbocation.
Let’s examine another example. In this case, unlike the previous one, we don’t initially have a good LG. However, we are working in acidic conditions, so let’s start with acid-base chemistry and see what develops. By protonating the -OH, we form a good leaving group!
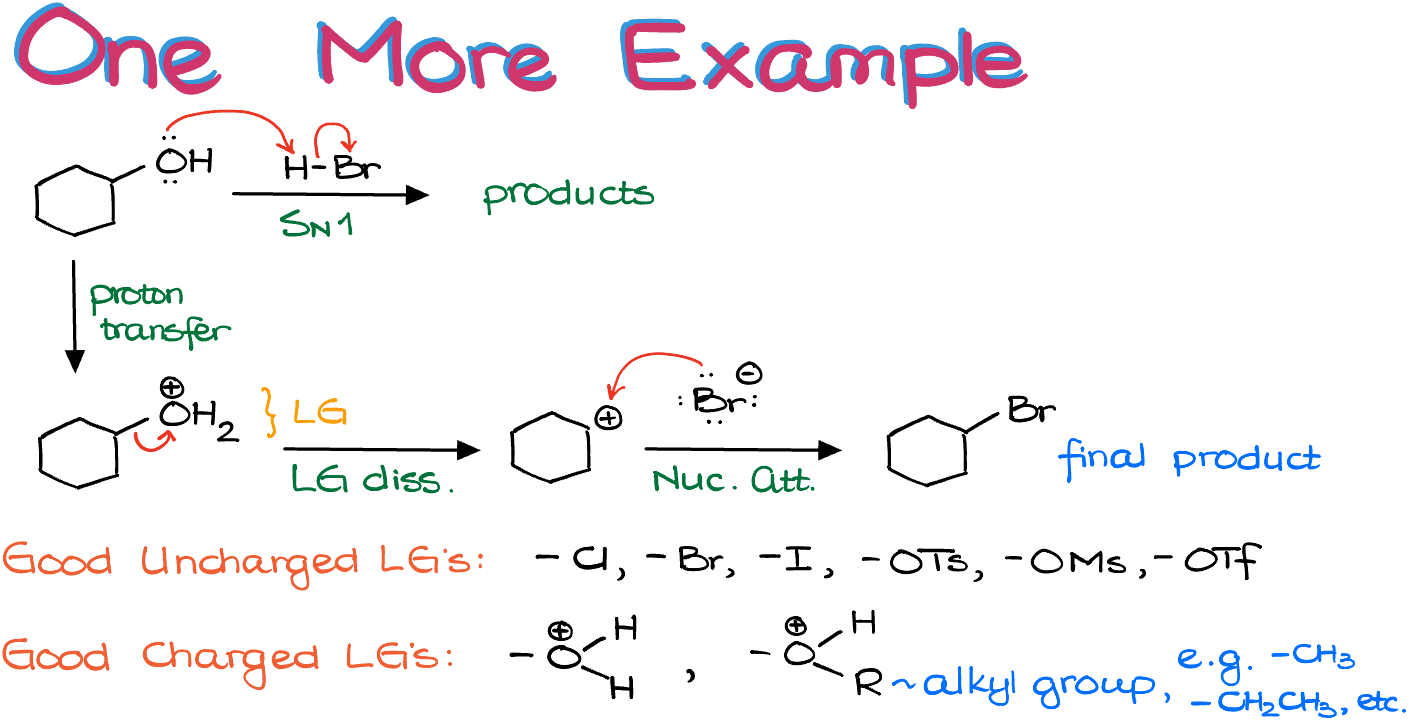
In addition to the six classic neutral leaving groups -Cl, -Br, -I, -OTS, -OMs, -OTf, we also have a few positively-charged LG’s.
Once we have a good leaving group, we proceed with its dissociation, forming a carbocation. Since carbocations are highly electrophilic (electron-loving), our carbocation will rapidly react with any available nucleophile. If we react with H2O, we return to the previous structure, so we avoid that. However, we have another nucleophile available – Br-.
If we plot the energy diagram for this reaction, we get the following:
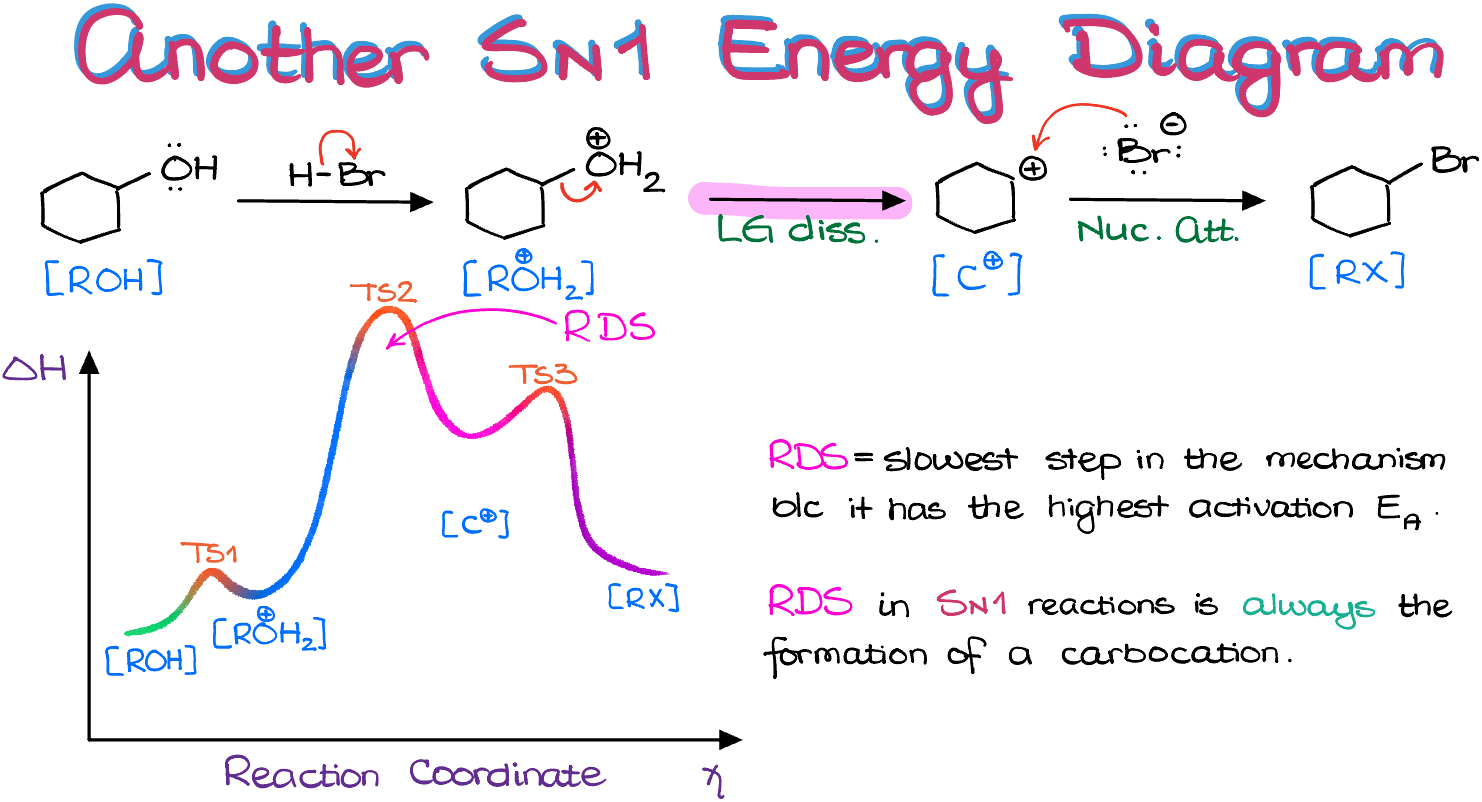
Notice that in this case, the RDS is the second step in our mechanism. So, it’s not the sequence of steps that matters, but rather what occurs in each step. In both examples covered in this tutorial, the RDS was the formation of a carbocation. As a general rule, the RDS is usually the step that forms the least stable intermediate in the reaction. Hence, in any reaction that produces a carbocation, the RDS will be the step forming the carbocation.
Stereochemistry of SN1 Reactions
Now, let’s discuss the stereochemistry of SN1 reactions. We know that SN2 reactions proceed with inversion of the stereoconfiguration, but what about SN1 reactions? Until now, our examples didn’t involve stereochemistry.
Consider this example:
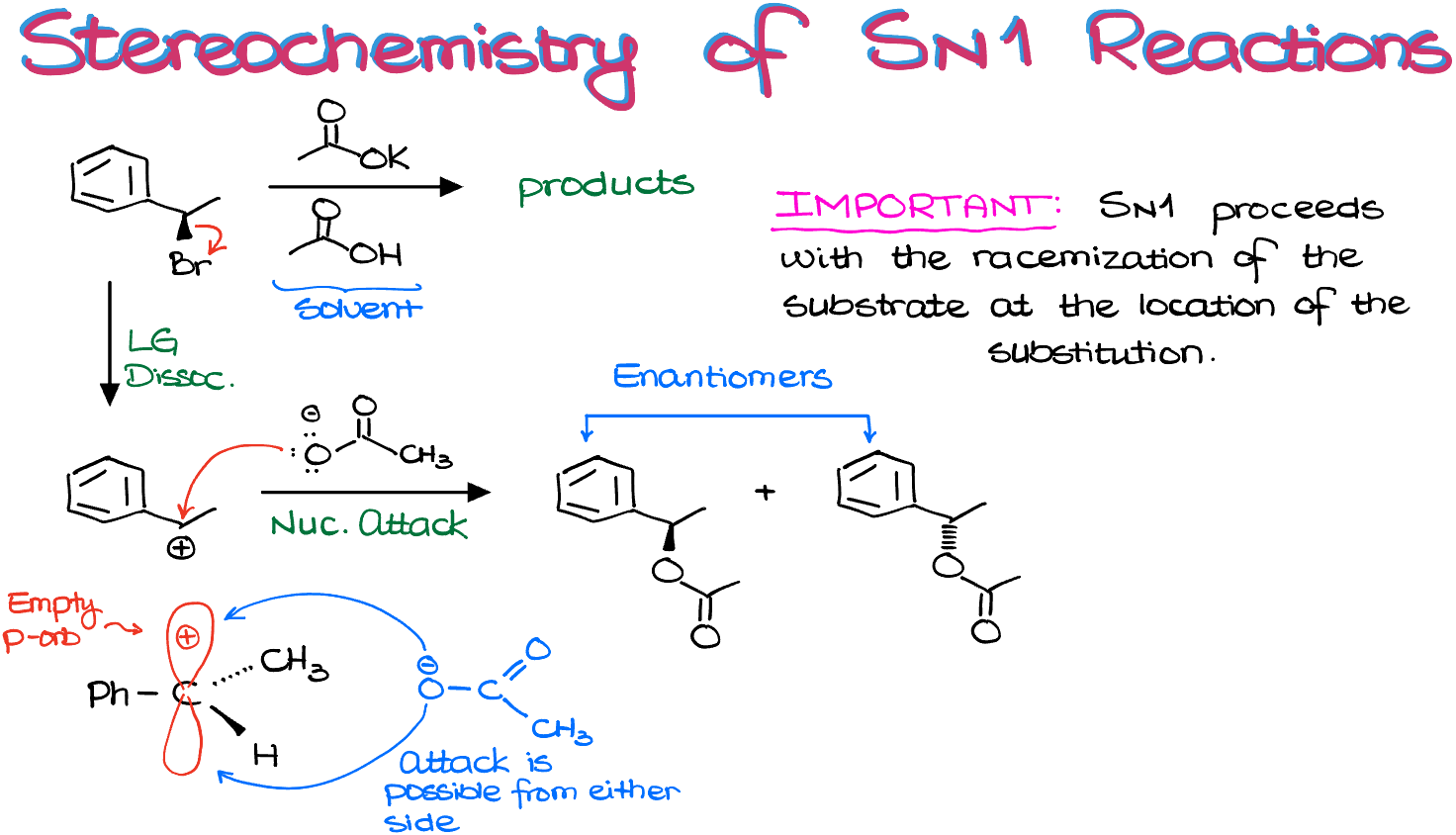
Walking through this mechanism step-by-step, we see that it forms a carbocation intermediate. As carbocations are sp2-hybridized and have trigonal planar geometry, the attack can occur from either side, leading to both possible stereoisomers as the product. Therefore, SN1 reactions result in racemization, or the loss of stereochemistry, and the formation of both potential stereoisomeric products.
Note: Similar to SN2 reactions with multiple stereocenters, SN1 reactions only racemize the atoms where the reaction takes place. For instance, take a look at this reaction:
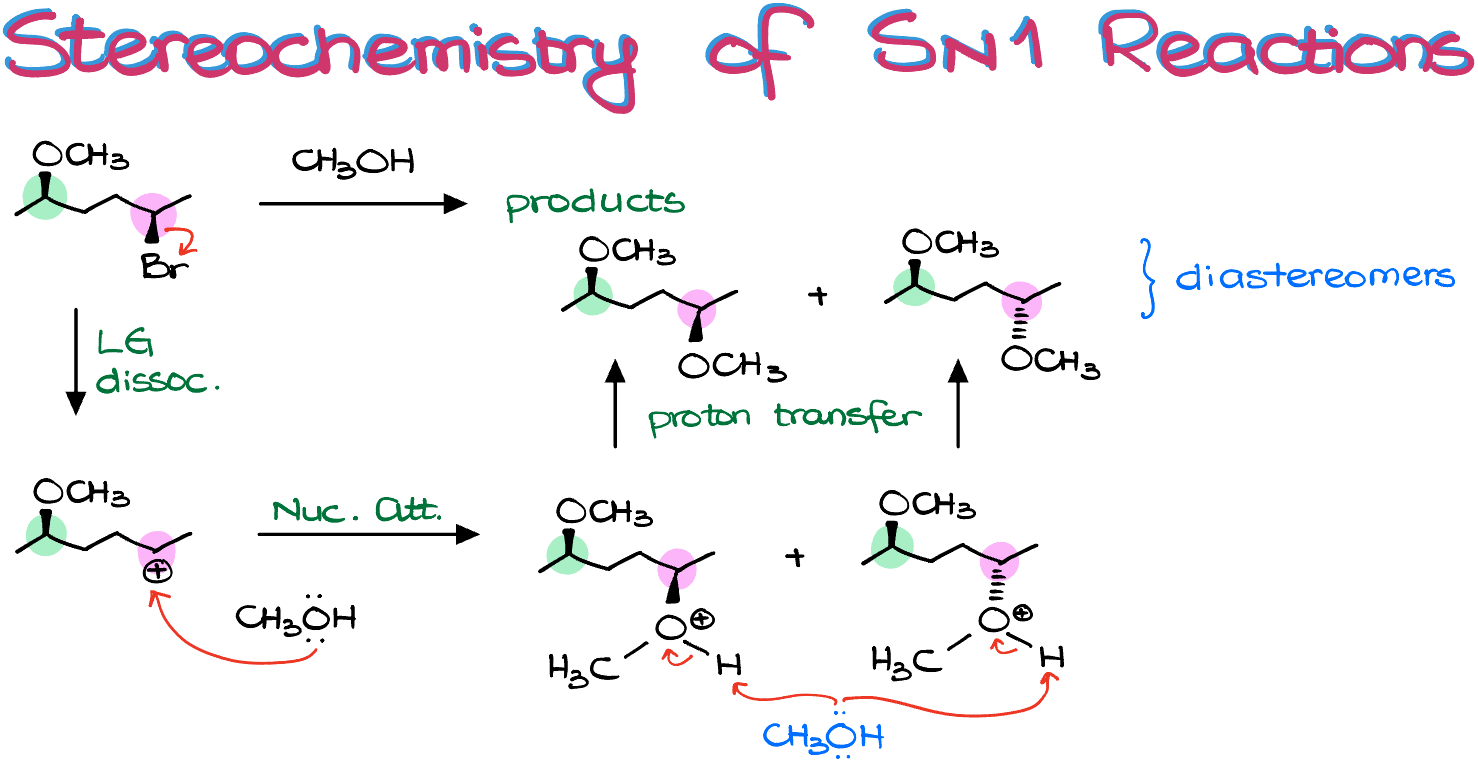
We obtain a pair of diastereomeric products because one of the chiral carbons remains unchanged while the other undergoes racemization. Always pay attention to your reaction mechanism before determining your final product.
Quick Recap of SN1 Reactions
Let’s quickly recap what we’ve learned about SN1 reactions:
- The reaction is first-order, represented as rate=k[Rx].
- It forms a carbocationic intermediate.
- The reaction results in a loss of stereochemistry.
- Polar protic solvents aid in the formation of carbocations and thus facilitate the reaction.

This tutorial doesn’t cover everything about SN1 reactions. Since we form carbocation intermediates, we can encounter various carbocationic rearrangements such as hydride and alkyl shifts. In the next tutorial, we’ll go over the basics of carbocation rearrangements and explain how to predict their outcomes.
Practice Questions
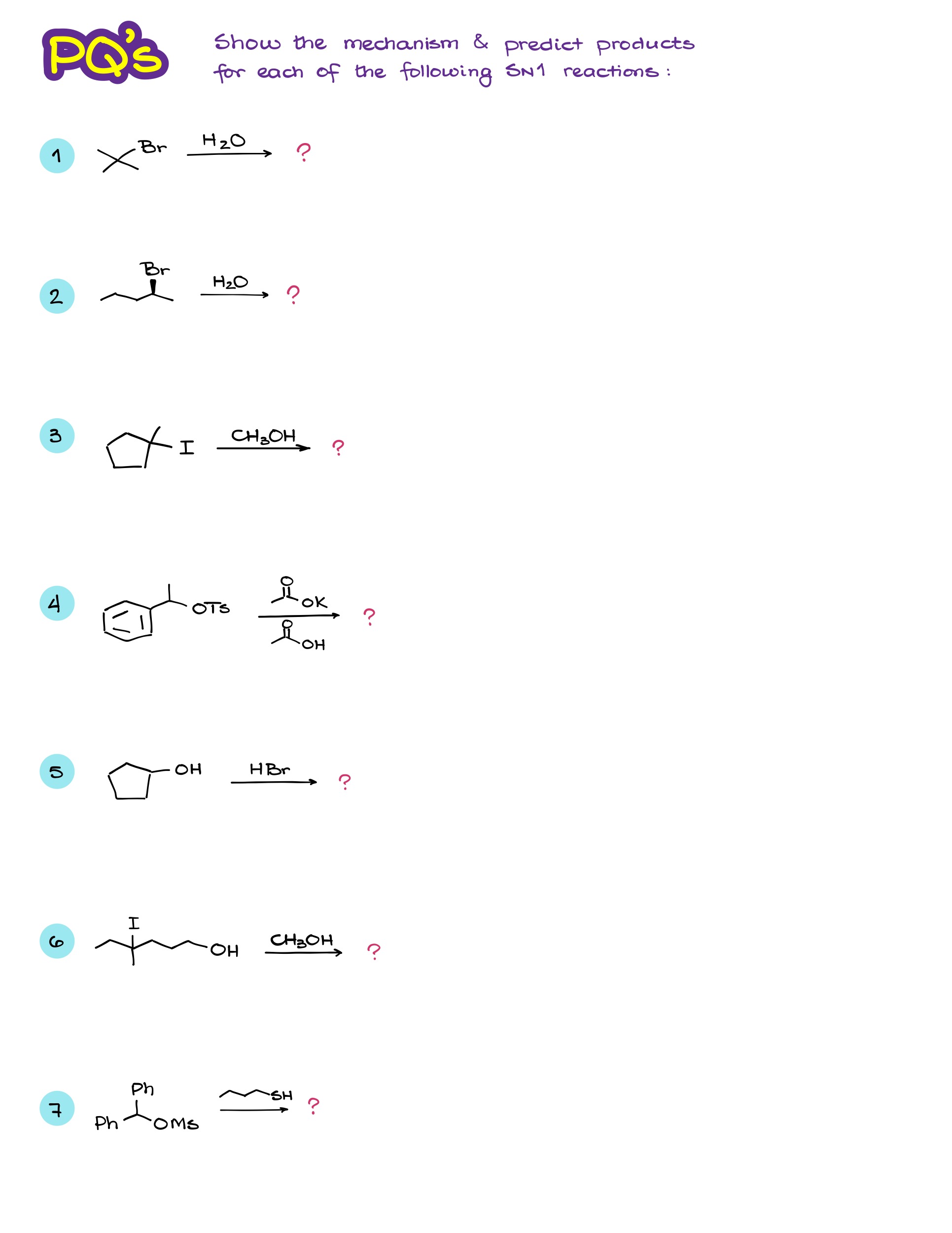
Would you like to see the answers and check your work? Become a member today or login if you’re already a member and unlock all members-only content!
