Friedel-Crafts Alkylation and Acylation Reaction
In this tutorial I wanna talk about the Friedel-Crafts reaction. When we say the Friedel-Crafts reaction, we typically refer to either the Friedel-Crafts alkylation or the Friedel-Crafts acylation reactions. And while they seem similar enough, there are a few important differences between them.
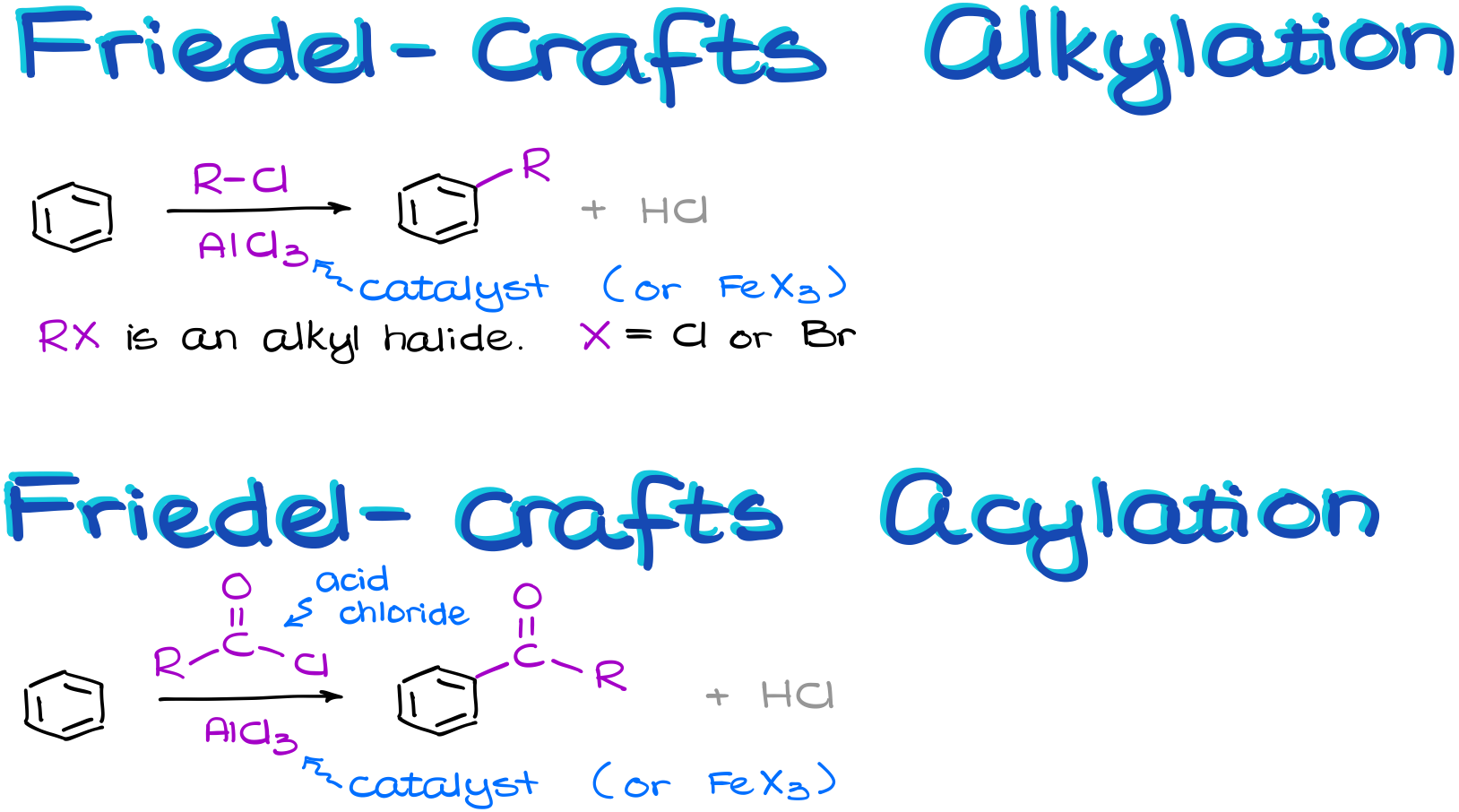
In the Friedel-Crafts alkylation reaction, we are going to perform a reaction between an aromatic compound and an alkyl halide. We’ll need to make sure that the leaving group we’re dealing with is on the sp3-hybridized atom. We’ll talk about the reasons for that once we look at the mechanism of this reaction in a few moments. Also, the halide is typically a chloride or a bromide. We’ll also need a Lewis Acid catalyst in this reaction. Typically, our catalyst is going to be either aluminum or iron halide.
The Friedel-Crafts acylation is somewhat similar. It also requires a catalyst like aluminum or iron halide.
However, instead of an alkyl halide, we react our aromatic compound with an acid chloride. And the nature of the R-group in this case is pretty much irrelevant. The acid chloride functional group is also occasionally called an “acyl chloride” thus the name for the reaction.
Mechanism of the Friedel-Crafts Alkylation
Let’s look at this reaction.
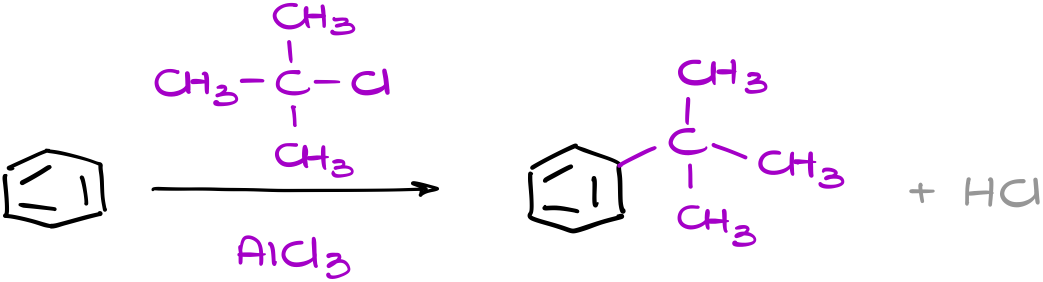
This is a typical example of the Friedel-Crafts alkylation. Here, we react and aromatic compound (benzene) with an alkyl halide (t-butyl chloride) and get a t-butylbenzene as a product.
Now, when it comes to the mechanism of this reaction, we are going to start like in any other electrophilic aromatic substitution with the formation of an electrophile.
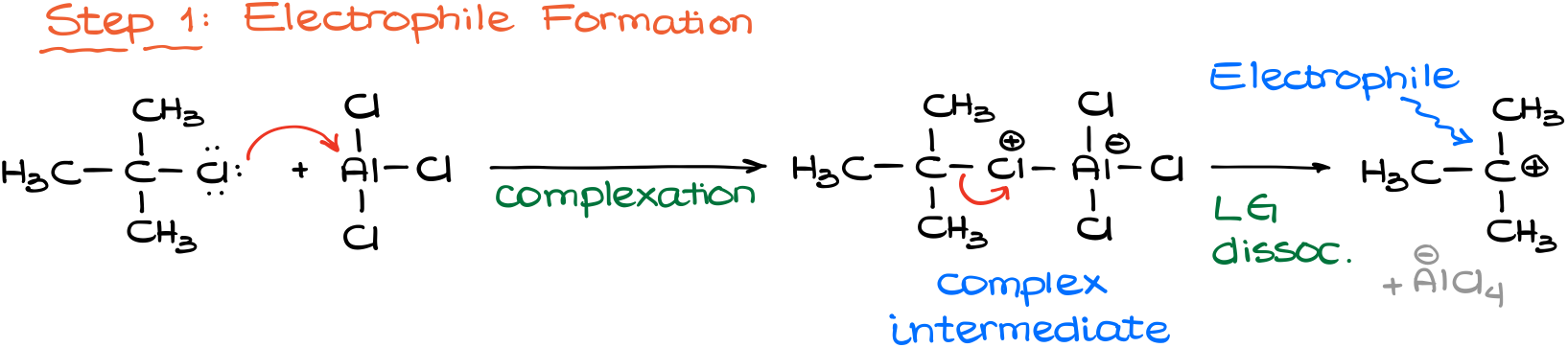
This is where our catalyst (aluminum chloride) comes in handy. As aluminum chloride is a strong Lewis Acid, it will coordinate with the chlorine of the alkyl halide giving us a complex intermediate, which will dissociate, essentially ripping the chlorine off out molecule forming a carbocation. This is also why having a halide on the sp3-hybridized atom is important. As carbocations are unstable, their ability to form is essential in this reaction. And we don’t typically see halides acting as a leaving group (even if we have a Lewis Acid tugging on it) when they are on a non-sp3-hybridized atom.
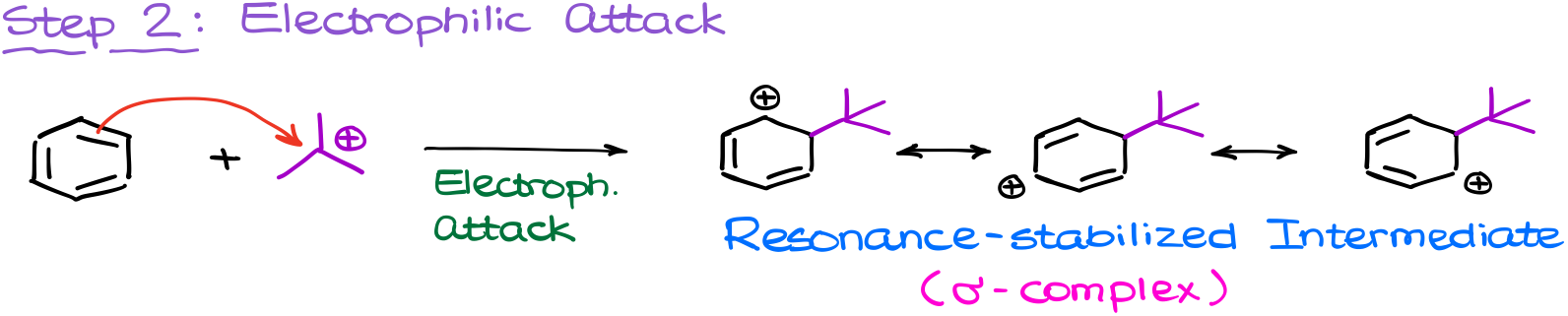
Next, the electrophile will attack the aromatic ring forming a resonance-stabilized carbocation intermediate, which we also know as a σ-complex.
And finally, we are going to restore aromaticity by removing the proton from the same position where we’ve added our electrophile. This step also forms our co-product, HCl, which we don’t care about, and regenerates our catalyst, AlCl3.
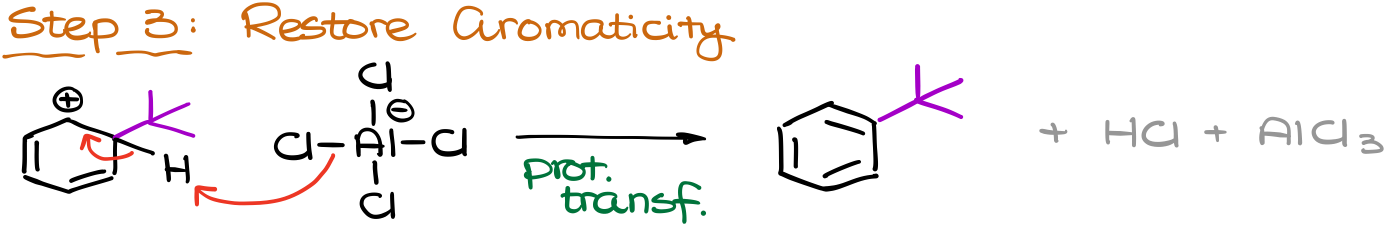
Sounds easy, right? BUT! We have a carbocation intermediate in this reaction and that means trouble! We know that carbocations can easily rearrange if given an opportunity to make a more stable carbocation.
Carbocation Rearrangements in Friedel-Crafts Alkylation Reactions
Suppose we have a reaction like this one.
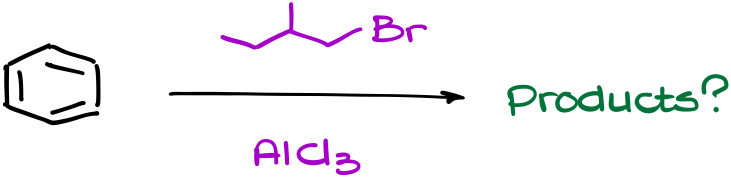
To find the correct answer to this question, we need to carefully work through eh mechanism of this reaction.
Let’s start by making a complex with our catalyst. This will give us the complex intermediate. And we know that the next step should be the complex dissociation giving us the carbocation. But if we do that, we’re going to end up with a primary carbocation, which is a huge no-no for us. Since primary carbocations are incredibly unstable, we’ll expect it to immediately rearrange to give us a more stable carbocation instead.
Alternatively, we can do the leaving group dissociation and the carbocation rearrangement happening simultaneously giving us the same tertiary carbocation and bypassing the formation of the primary carbocation altogether. Research evidence suggests that in majority of the cases, the second pathway of the simultaneous dissociation-rearrangement is more likely. So, even if we do end up forming some of the primary carbocation, its concentration is going to be truly negligible.
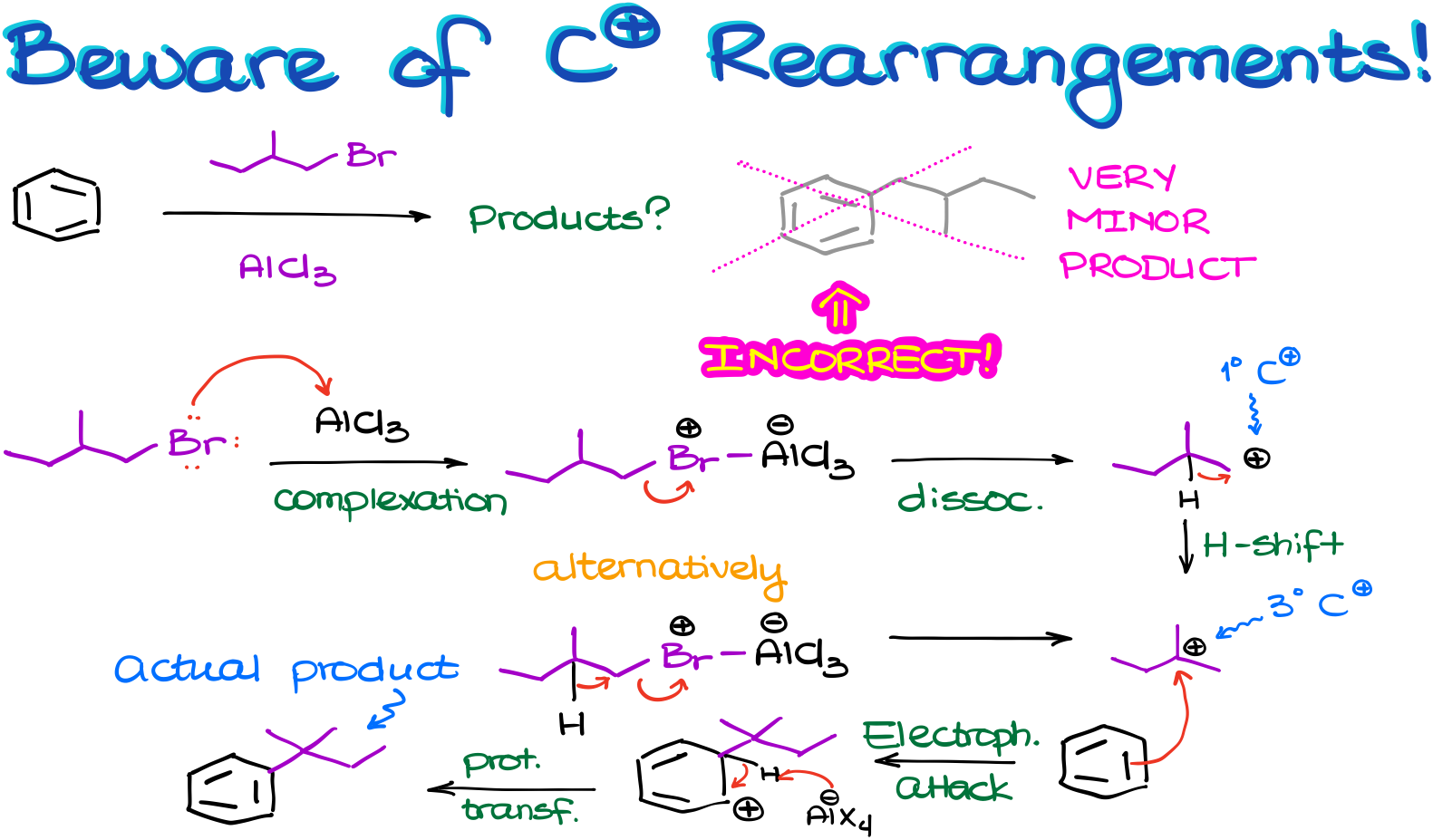
Next, once we have our actual electrophile in this reaction, we are going to do the electrophilic attack on the aromatic ring.
And so, the last step in our mechanism is now going to be the restoration of aromaticity via a proton transfer.
The carbocation rearrangements in the Friedel-Crafts alkylation reactions are a classic complication. And you can be sure your instructor is going to add one of those reactions on the test to see if you remember that or not. I said it before, and I’ll repeat it again: if you form a carbocation, always check for possible carbocation rearrangements. If you feel shaky on those, it might be a good idea to refresh your memory now and make sure you are coming to the test all ready.
Friedel-Crafts with Primary Alkyl Halides
Now, what if we have a molecule where the primary carbocation would not be able to rearrange and give you something more stable? Does the reaction fail then?
Actually no.
If we have a situation where the rearrangement is impossible, like in the case of the methyl and ethyl halides, the reaction still occurs. However, we’re not going to form a carbocation and the reaction will proceed via the SN2 mechanism.
For instance, in this example, we have a reaction between benzene and bromomethane in the presence of iron bromide catalyst.
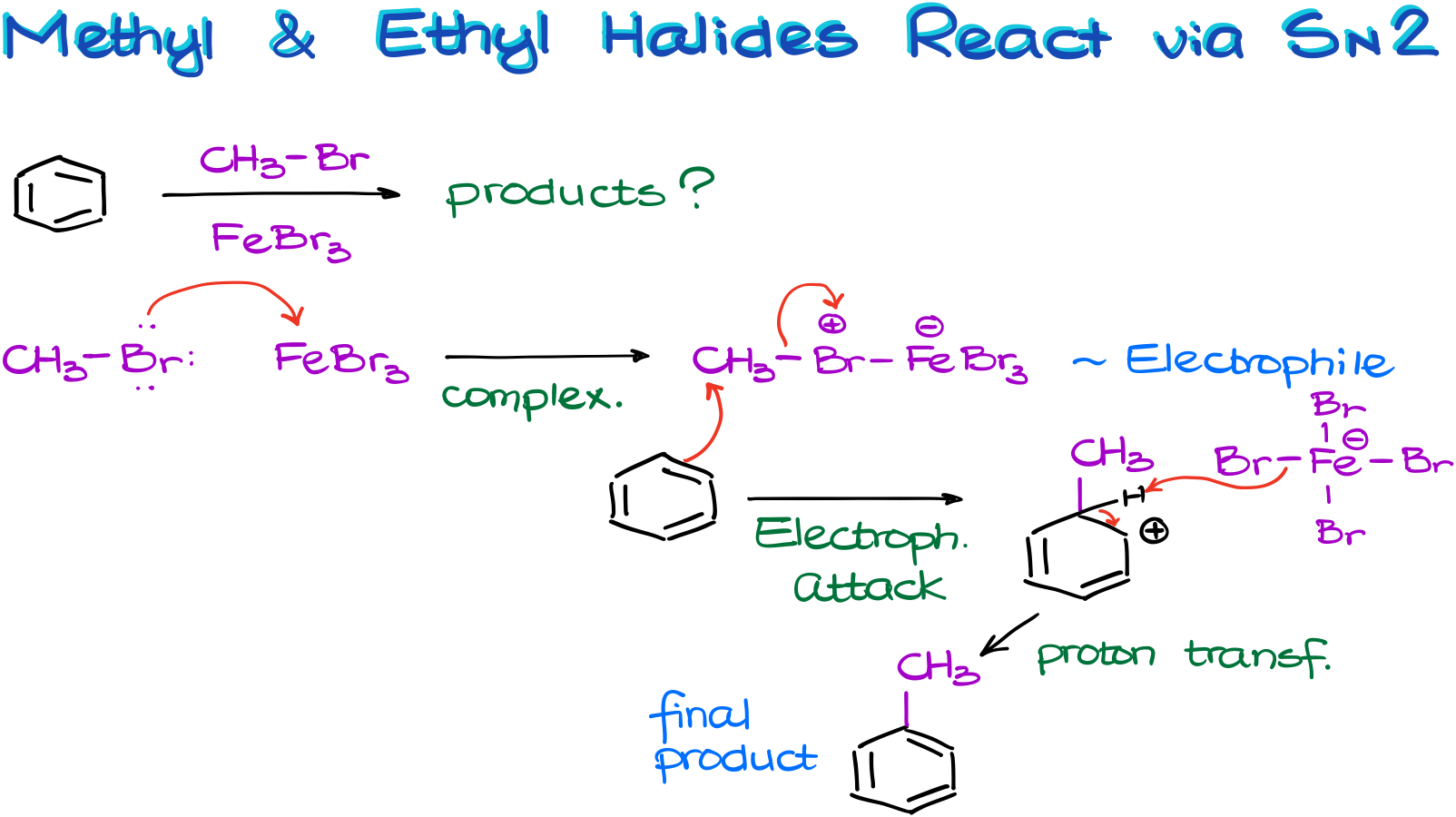
As the carbocation formation here is nearly impossible due to the instability of the methyl and primary carbocations, the complex with the metal will be our electrophile.
And the aromatic compound will attack the carbon directly via the SN2-style mechanism. We’re still going to make our usual resonance-stabilized σ-complex. I’m not showing all the resonance structures here, but you should still draw them if you need to show this mechanism on the test.
Finally, once we remove the proton from our carbocation intermediate and restore the aromaticity, we get our final product.
So, as you can see, the reaction works just fine, but the mechanism is a little different from what we’d normally expect.
Multiple Alkylations in the Friedel-Crafts Alkylation Reaction
And talking about the alkyl groups, they bring another complication to the table. Here’s a thing, an alkyl group is—what we would call it—an activating group. And what that means is that the product of our reaction is going to be more reactive than the starting material.
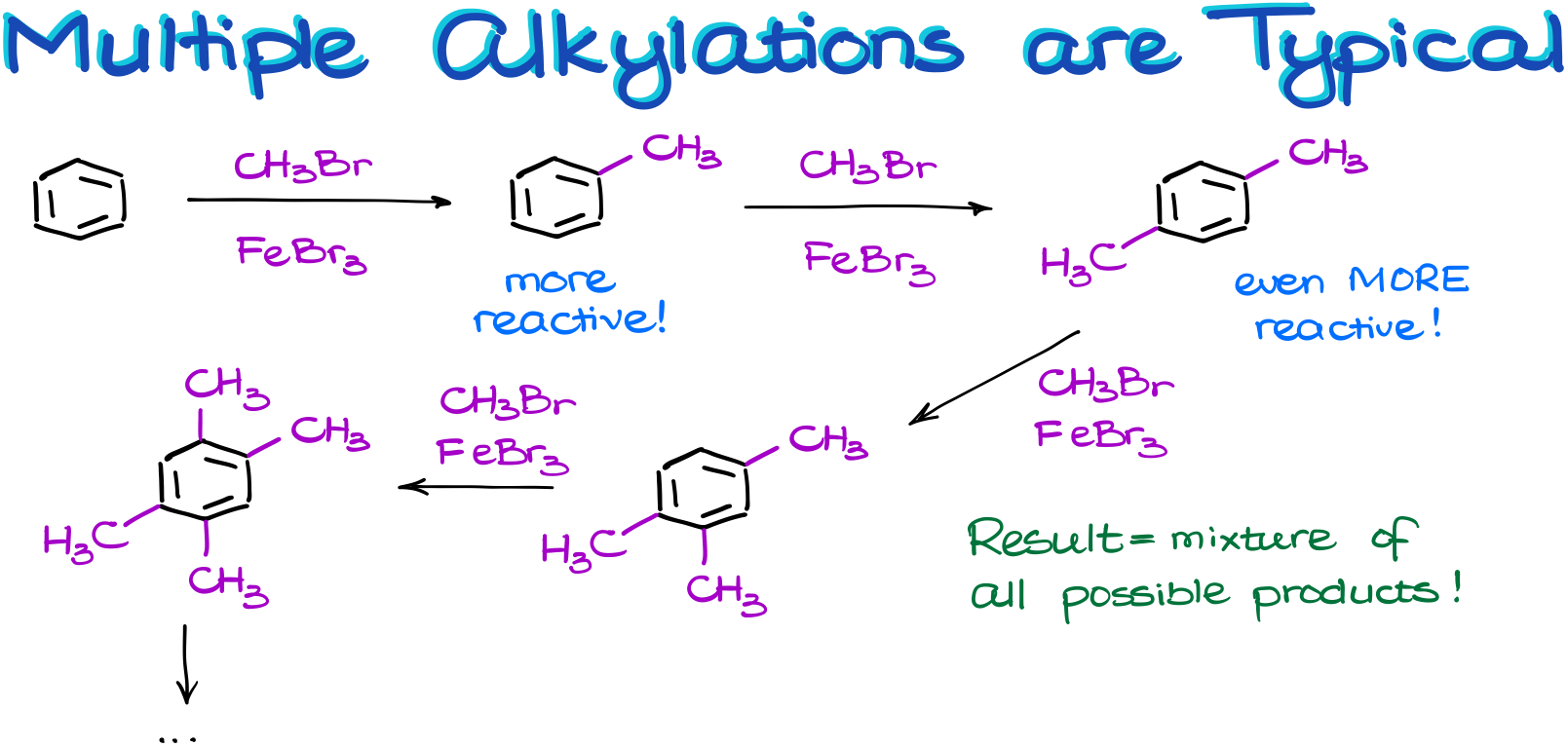
So, in this reaction, the product will try to react with our electrophile more aggressively than the starting material.
Remember, that when we are performing a reaction, we never have a situation in which all our molecules react simultaneously. We only have some portion (usually rather small) of our molecules reacting while the majority are just chilling around minding their own business. So, once we have the first crop of our product in the solution, we still have plenty of unreacted starting materials and reagents. And that’s where the problem is.
So, we end up with a soup of all possible products in some ratios. Thus, we almost never perform this reaction in the lab as it would be a huge waste of materials. Not to mention, that the product purification will be a complete nightmare. This reaction is, however, routinely done on an industrial scale where the formation of the multiple products is not an issue. When you have a reaction mixture that’s a few thousands of liters in size, you can do a huge industrial-scale distillation and separate your products. Just like what they to in petroleum industry distilling different types of fuel in enormous distillation setups. Doing something like that in the lab would not be economical.
Some instructors are ok with you doing a reaction like that on the test though. So, I would encourage you to double check with your instructor if this reaction is a “go” when it comes to the synthesis, or this reaction is a “no-go” for the test purposes. I can debate all day long about “paper chemistry,” aka chemistry that only exists on paper and not in real lab, but at the end of the day, it’s your instructor who’s going to be giving you your grade. So, go with what they allow you to do in their class. In my experience, about half of the instructors will not consider the Friedel-Crafts alkylation as a viable method of adding a simple alkyl group to your aromatic molecule. Some more complex situations are a fair game though.
Different Ways of Making a Carbocation
Talking of performing this reaction in the lab, it actually doesn’t matter how you make your carbocation. Let me illustrate.
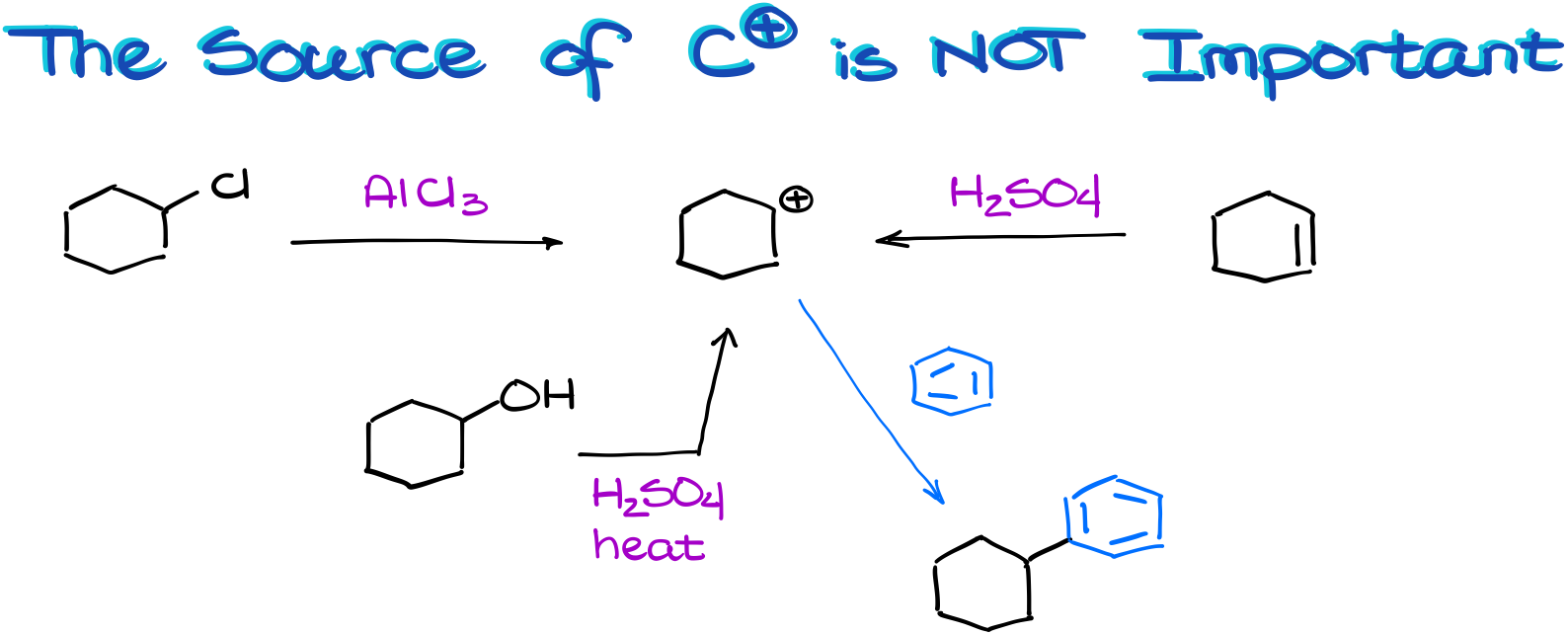
We can make the same carbocation through the complexation with a Lewis Acid. Or we can make it by protonating an alkene. Or we can make it by dehydrating an alcohol! So, remember, if you end up with a carbocation and an aromatic compound, chances are, they are going to react with each other regardless of where that carbocation came from or how you made it!
In this case, for instance, we could have made our carbocation via three different starting materials. And yet, we still ended up with the same product. So, don’t lock yourself in a box by thinking that the Friedel-Crafts reaction can only work for alkyl halides.
Friedel-Crafts Acylation
Alright. Now, how about the Friedel-Crafts acylation reaction? The reaction is somewhat similar to the Friedel-Crafts alkylation. But instead of an alkyl halide we use the acid halide (also called an acyl halide). We also use a Lewis Acid catalyst like aluminum chloride in this reaction.

So, let’s look at the mechanism of this reaction and see what the difference between the alkylation and the acylation reactions is.
Mechanism of the Friedel-Crafts Acylation Reaction
Like in any electrophilic aromatic substitution, we start by making an electrophile.
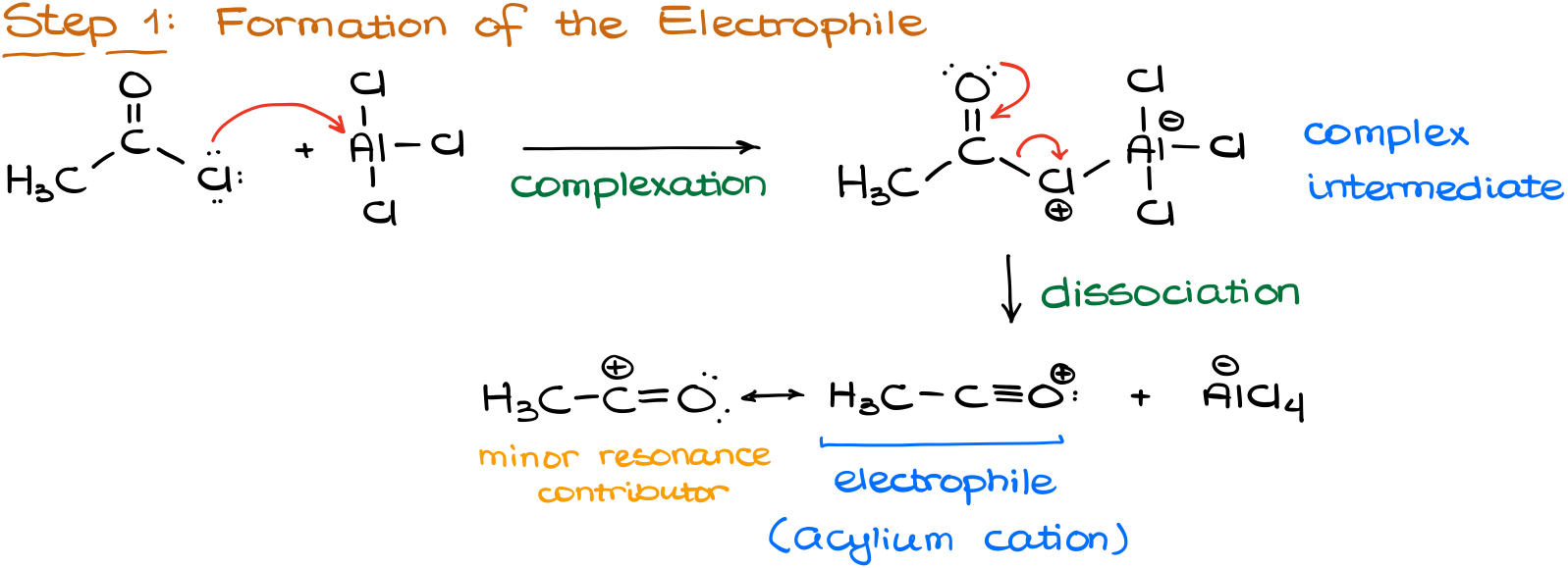
In the alkylation reaction, we start by making a complex between our reagent (acyl halide) and the catalyst (aluminum chloride). This complex then can undergo dissociation giving us the electrophile. And you are probably thinking, “wow, dude! Didn’t you just say that we have to have our leaving group on an sp3-hybridized atom?” And yes, I did, BUT! This electrophile is resonance stabilized. Thus, it’s actually pretty stable and will form quite easily.
Additionally, because it is resonance stabilized, we are not going to be expecting any kind of carbocation rearrangements.
Once we have our electrophile, it’s time for the electrophilic attack on the aromatic ring.
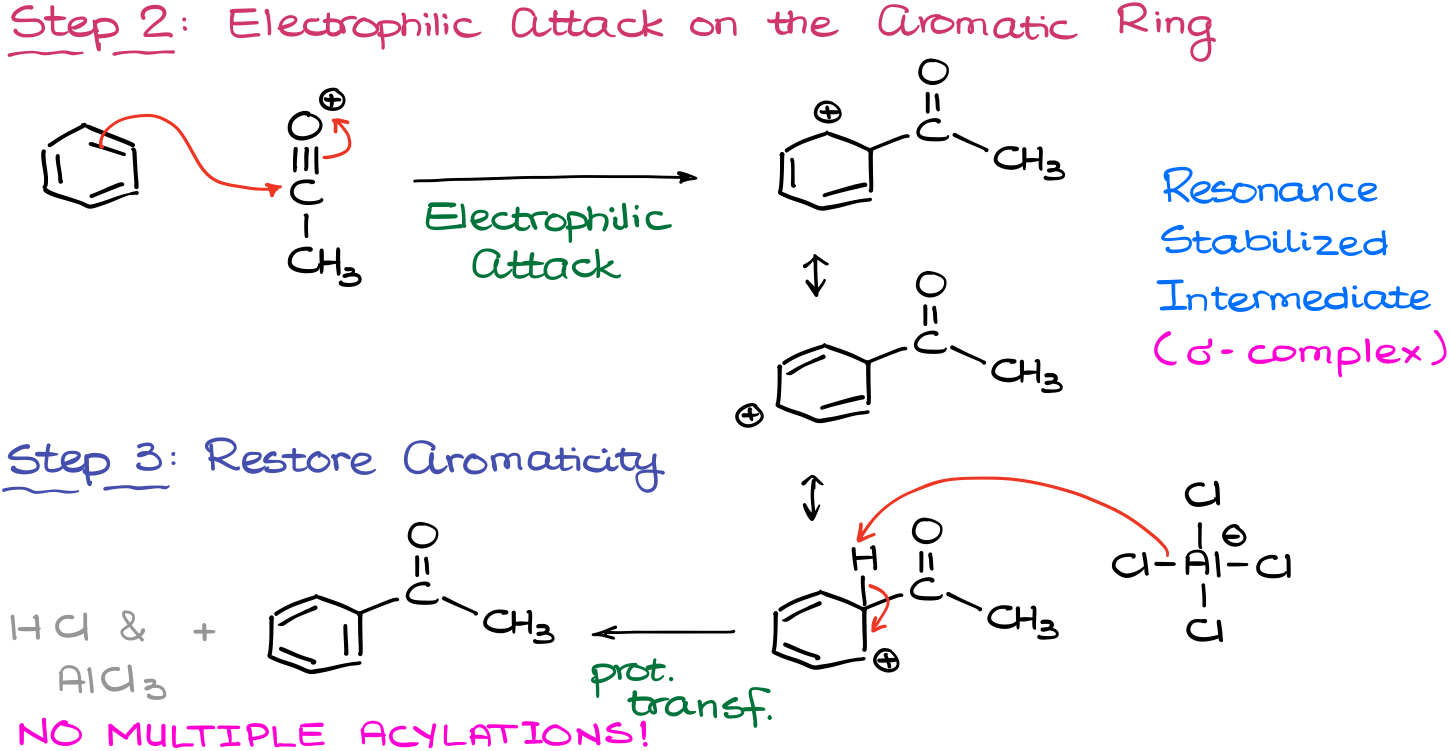
As the result of this attack, we are going to see the formation of the resonance stabilized carbocationic species, which we know as a σ-complex.
Finally, we’ll restore aromaticity by elimination of the proton from the position of the electrophile’s attachment. As a base for this proton transfer, we are going to use the aluminum chloride complex just like in the alkylation reaction. We’ll also form the co-product (HCl) in this step and regenerate our catalyst.
So, as you can see, the mechanism is pretty much the same, minus the carbocation rearrangements.
There’s another advantage of using the Friedel-Crafts acylation over the alkylation. The introduction of the carbonyl as a substituent on the aromatic ring makes the compound significantly less reactive towards electrophiles. Thus, we are not going to see multiple acylations in this case unlike what we saw with the alkylations. Because of that the reaction is significantly easier to control. This is, however, a limitation of the Friedel-Crafts acylation and alkylation as well: the reaction fails when we try to perform it on the deactivated aromatic rings.
Review of the Friedel-Crafts Alkylation and Acylation Reactions
Alright, let’s review what we’ve learned today!
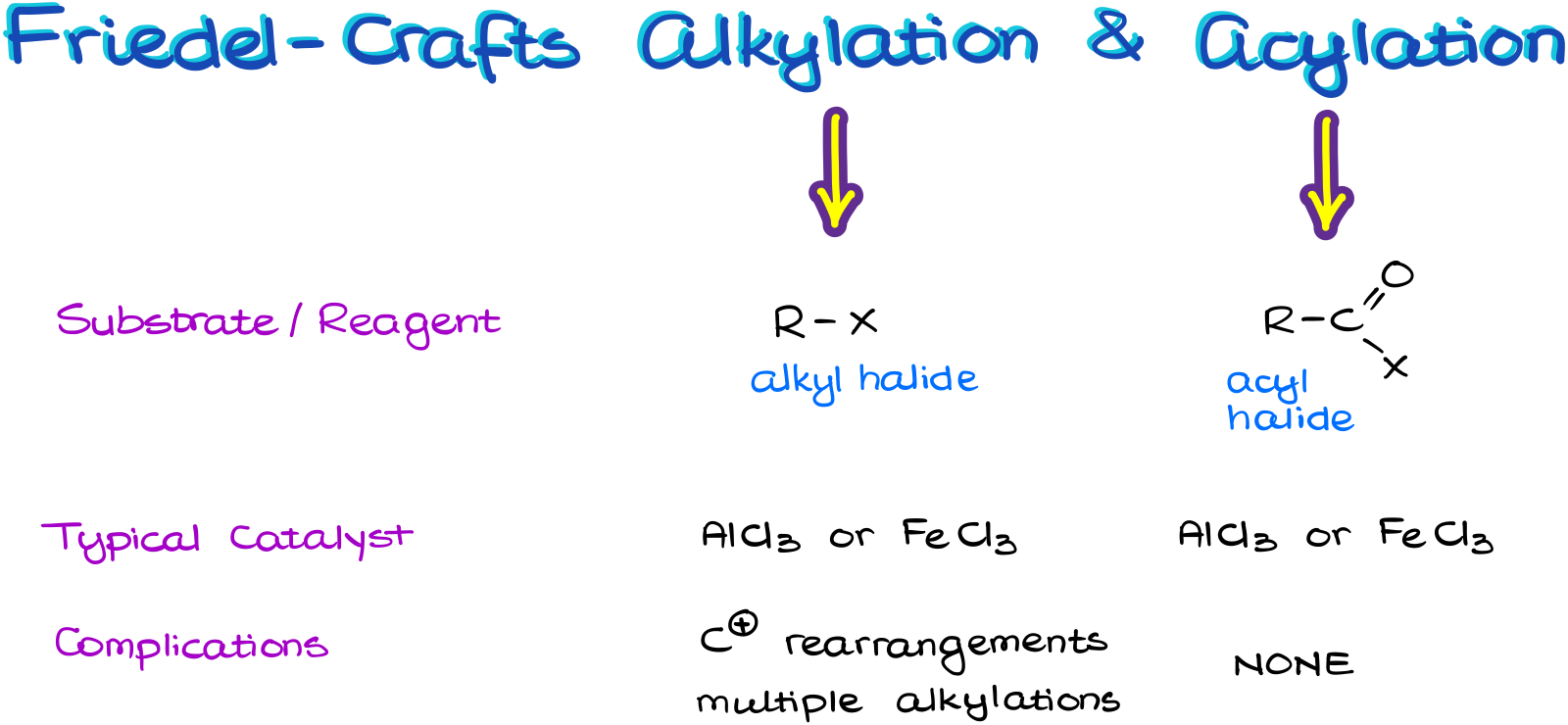
We use the alkyl halide for the alkylation and acyl halide (aka, acid halide) for the acylation reaction.
We typically see the same Lewis Acid catalyst for this reaction. Most commonly, it is going to be the aluminum chloride.
And we also need to be aware of the carbocation rearrangements and possible multiple alkylations in the Friedel-Crafts Alkylation reaction. Luckily, the acylation does not have this problem. So, when possible, we are going to be using the acylation reaction.
