Mechanism Challenge: Moving Ester
Here’s an interesting mechanism challenge questions I got from one of my students. While it was not originally a multiple-choice question, I converted it to one based on our conversation and some common mistakes students can make while working through it.

We’ll start by analyzing our reagents.
Sodium hydride is a very powerful base. Like in many cases with the introductory organic chemistry topics, the sodium ion’s participation in this reaction can be completely neglected.
As I’ve mentioned a moment ago, hydrides are powerful bases. It’s also important to note, that simple hydrides like sodium, potassium, or lithium hydrides are not particularly nucleophilic. Thus, I’m not going to be considering any kind of nucleophilic substitution with the hydride ion in this reaction.
So, since the hydride ion is a base, I’m going to look for the acidic protons in my molecule. I have two possible contestants here. By quickly checking the pKa table, we can find that the pKa values are quite different for those. The alcohol has a typical pKa about 16 to 18, while the ɑ-position of esters is somewhere around 21. And while we don’t know the exact pKa values for this particular molecule, the typical values are a good enough estimation for our purposes.
Since the difference in our pKa values is quite significant (the difference of 3 units means we have 1000 times difference in acidity), we can safely say that the deprotonation of the ɑ-position is going to be negligible compared to the deprotonation of the alcohol.
So, I’m going to start my reaction by deprotonation of my alcohol making the corresponding alkoxide.
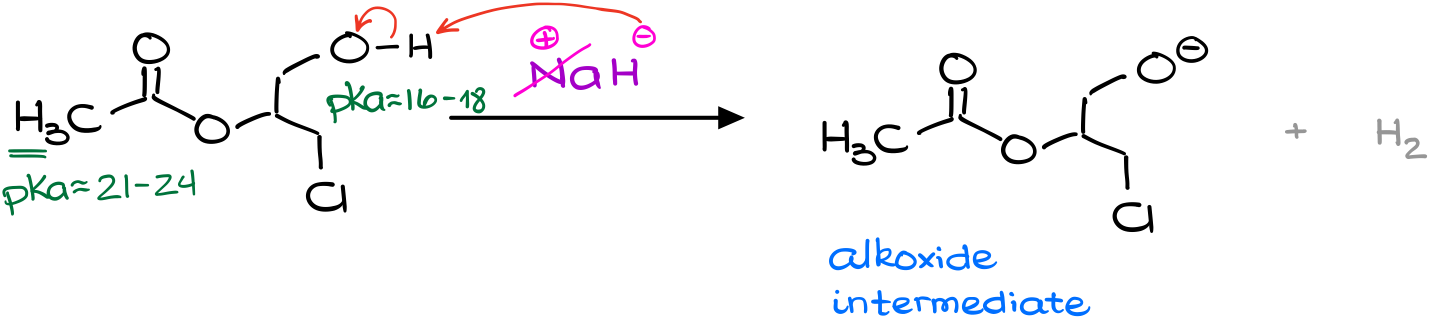
From this point, I have two possible options of what might happen next.
First, we can have an attack by our nucleophile on the electrophilic carbon attached to the chlorine atom.
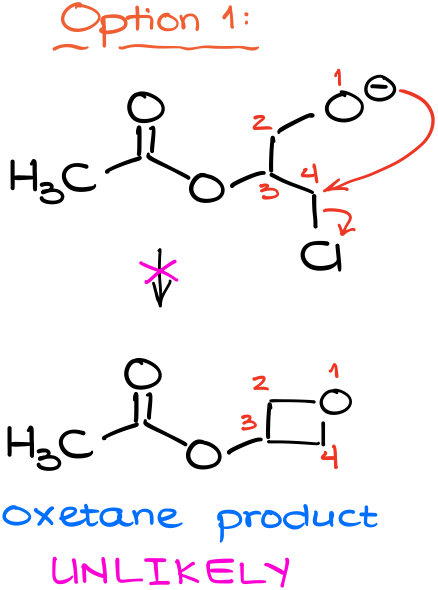
This would yield a 4-membered ring with oxygen, we call molecules like that an oxetane. And while theoretically reactions like that are possible, they are exceptionally rare and oxetanes typically do not form like that. As a rule of thumb, always remember, that making a 4-membered ring is a very rare and unfavorable process in the vast majority of cases, and we only see these reactions in exceptional cases. So, in other words, this pathway is unlikely.
Another option would be the attack on the ester group.
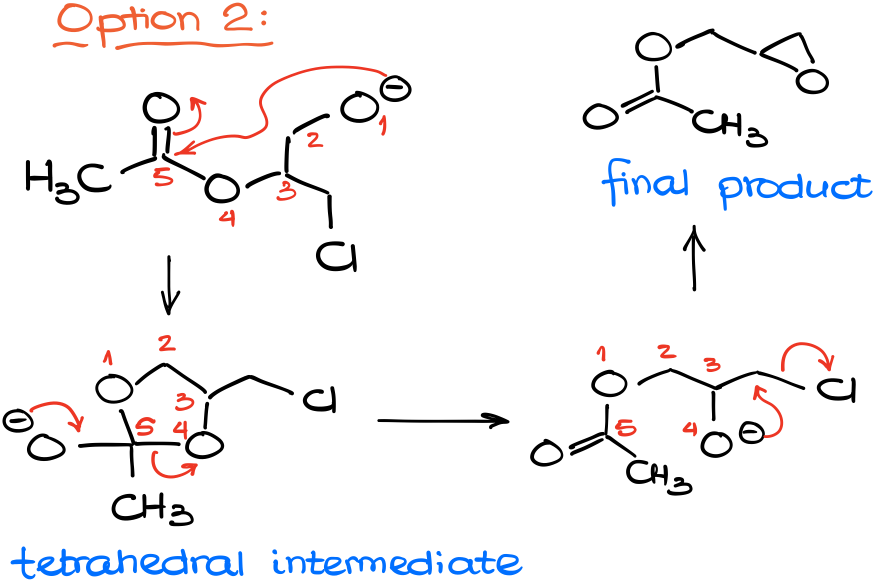
Which results in a tetrahedral intermediate characteristic for the chemistry of carboxylic acids and their derivatives. And as this is a 5-membered ring intermediate, there are no problems with its formation.
And as our tetrahedral intermediate pushes the leaving group out to restore the carbonyl, we break the C-O bond between the atoms 4 and 5.
This, essentially, “moves” the acyl group from one oxygen of our molecule to another one. Now we have a nucleophilic oxygen right next to an electrophilic carbon with a leaving group. And unlike the 4-membered ring, 3-membered rings close very easy due to the proximity of the reactive centers.
Giving us the resulting epoxide as the final product.
Did you get the same product? Let me know in the comments below!
